Acticon. Neosphincter Instructions for Use. Acticon page 1 Neosphincter Instructions for Use. Acticon page 19 Néosphincter Mode d emploi
|
|
|
- Gabriel Coronel Lara
- hace 8 años
- Vistas:
Transcripción
1 Acticon Neosphincter Instructions for Use Acticon page 1 Neosphincter Instructions for Use Acticon page 19 Néosphincter Mode d emploi Acticon page 37 Neosphinkter Gebrauchsanleitung Acticon page 57 Neosfintere Istruzioni per l uso Acticon page 75 Neoesfínter Instrucciones de uso Acticon page 95 Neo-esfíncter Instruções de Utilização CAUTION: Federal law (U.S.) restricts this device to sale by or on the order of a physician.
2 European Union (EU) Authorized Representative: American Medical Systems Europe B.V. Straatweg 66H 3621 BR Breukelen The Netherlands Tel: + 31 (0) Fax: +31 (0) Contact List: American Medical Systems U.S.A Bren Road West Minnetonka, MN U.S.A. Tel: Fax: American Medical Systems Australia Pty Ltd. Unit 39, Building F 16 Mars Road Lane Cove NSW 2066 Australia Tel: Fax: American Medical Systems Canada Inc. P.O. Box 461 Guelph, Ontario N1H 6K9 Canada Tel: Fax: American Medical Systems Deutschland GmbH Voßstr. 20 D Berlin Germany Tel: + 49 (0) Fax: +49 (0) American Medical Systems France 19 avenue de Norvège Les Fjords - Bâtiment Nobel Courtaboeuf Cedex France Tel: + 33 (0) Fax: + 33 (0) American Medical Systems Ibérica S.L. c/joaquin Turina, 2 Planta primera - Oficina Pozuelo de Alarcón (Madrid) Spain Tel: + 34 (0) Fax: +34 (0) American Medical Systems U.K. Ltd. Capital Court Capital Interchange Way Brentford TW8 0EX United Kingdom Tel: + 44 (0) Fax: + 44 (0)
3 English Instructions for Use AMS Acticon Neosphincter NOTE: Refer to the Operating Room Manual for further information on the Acticon Neosphincter and its implantation. BRIEF DEvICE DESCRIPtIoN The Acticon Neosphincter is an implant able, fluid-filled, solid silicone elastomer device used to treat severe fecal inconti nence. Severe fecal incontinence is defined as the involuntary loss of liquid or solid stool more than once a week. The Acticon Neosphincter may be implanted in either women or men and consists of three interconnected com ponents: an occlusive cuff, a pressure-regulating balloon and a control pump with a septum. The three components are connected with kink-resistant tubing. The Acticon Neosphincter simulates normal anal sphincter function by allow ing the anal canal to open at the control of the patient. The occlusive cuff is implanted around a segment of the anal canal. The device maintains continence in the patient by using the pressure of the fluid-filled cuff to occlude the anal canal. To evacuate the bowel, the patient squeezes and releases the pump mechanism, located in the labium or scrotum, several times to move fluid from the cuff to the pressure-regulating balloon implanted in the prevesical space. This movement of fluid empties and collapses the cuff, resulting in the release of the compressive force around the anal canal. Residual pressure within the balloon allows fluid to flow back into the cuff, automatically refilling the cuff within a few minutes. The pressure-regulating balloon maintains pressure in the occlusive cuff. This device contains solid silicone elastomer. INDICAtIoNS FoR USE The Acticon Neosphincter is an implant able device used to treat severe fecal incontinence in males and females eighteen years and older who have failed, or are not candidates for, less invasive forms of restorative therapy. 1
4 CoNtRAINDICAtIoNS 1. This device is contraindicated in patients whom the physician deter mines to be poor candidates for surgical procedures and/or anesthesia due to physical or mental conditions. 2. This device is contraindicated in patients with fecal incontinence com - plicated by an irreversibly obstructed proximal segment of bowel. 3. This device is contraindicated in patients with an active infection. WARNINGS 1. Patients with diabetes, spinal cord injuries, musculoskeletal abnormali - ties, pre-existing stomas, or open sores in the region of the surgery have an increased risk of infection associated with a prosthesis. Patients who are immunocompromised or immuno supressed may also be at a higher risk for infection associated with a prosthesis. Appropriate measures should be taken to reduce the likelihood of infection. Infection that fails to respond to antibiotic therapy may result in removal of the prosthesis. Infection followed by explantation of the device may result in scarring which may make subsequent reimplantation more difficult. 2. Erosion may be caused by infection, pressure on the tissue, improper cuff sizing, improper balloon selection, tissue damage, and component misplacement. The cuff may erode around the anal canal or through the perineal skin. The control pump may erode through the scrotal or labial skin. The pressure-regulating balloon can erode into the bladder. Failure to evaluate and promptly treat the erosion may result in a substantial worsening of the condition leading to infection and/or loss of tissue. 3. This device contains solid silicone elastomers. This device does not contain silicone gel. The risks and benefits of implanting this device in patients with documented sensitivity to silicone should be carefully considered. 4. Surgical, physical, psychological, or mechanical complications, if they occur, may necessitate revision or removal of the prosthesis. Removal of the device without timely reimplanta tion of a new device may complicate subsequent reimplantation. The timing of reimplantation should be deter - mined by the treating physician based on the patient s medical condition and history. 2
5 PRECAUtIoNS Patient-Related Precautions 1. Patient selection requires thorough preoperative consultation and evaluation by the physician. 2. Patients should be counseled in order to have a realistic expectation of the physical, psychological, and functional outcome of the implantation of an Acticon Neosphincter. Although the prosthesis is designed to restore bowel control, some patients continue to have a degree of incontinence after this procedure. 3. Patients may experience pain when the device is activated in the postoperative period and during periods of initial use. Cases of chronic pain associated with the device have been reported. Pain with a severity or duration beyond that which is expected may require medical or surgical intervention. Patients should be counseled on expected postopera tive pain including severity and duration. 4. Tissue fibrosis or previous surgery in the area of the implant may preclude implantation of an occlusive cuff at the anal canal. 5. Acute bowel disorders, e.g. diarrhea or constipation, can interfere with proper functioning of the device and may require the use of external pads or manipulations to assist defecation. 6. Any progressively degenerative disease, e.g. multiple sclerosis, may limit the future usefulness of the implanted prosthesis as a treatment of the patient s fecal incontinence. 7. Adequate manual dexterity, strength, and motivation are required for proper use of the device. 8. Trauma or injury to the pelvic, perineal or abdominal areas, such as impact injuries associated with sports, can result in damage to the implanted device and/or surrounding tissues. This damage may result in the malfunction of the device and may necessitate surgical correction including replace ment of the prosthesis. The physician should advise patients of these possibilities and warn them to avoid trauma to these areas. 9. If a radiopaque solution is used instead of sterile isotonic saline to fill the device, ensure the patient is not allergic to the radiopaque solution. 10. Receptive anal intercourse may damage the occlusive cuff and is not recommended for patients implanted with this prosthesis. 11. Vaginal delivery of children may interfere with future proper function ing of the occlusive cuff. Patients should be made aware of the need to discuss the presence of the Acticon device with their doctors should they become pregnant. A Cesarean delivery may be recommended in order to avoid damage to the device. 3
6 12. No safety or effectiveness data exists for patients with a history of inflamma tory bowel disease or pelvic radiation. The potential for increased risk of erosion in these patients is unknown. Surgery-Related Precautions 1. Improper cuff sizing, improper balloon selection or other causes, such as surgical trauma, poor tissue viability, and concomitant medical procedures, may result in tissue erosion, migration of components, or continued incontinence. 2. Component migration can occur if the cuff is sized improperly, if the pump or balloon is not positioned correctly, or if the tubing lengths are incorrect. Migration can result in pain, complications, device malfunction and surgical revision. 3. Unsuccessful outcomes may result from improper surgical technique, anatomical misplacement of components, improper sizing and/or filling of components. 4. Although reinforced tubing has been designed to be more resistant to tubing kinks, tubing kinks may still result from tailoring the connecting tubing to an improper length during the implant procedure. Device-Related Precautions 1. This device is subject to wear and eventual failure over time. It is not possible to predict how long the implanted prosthesis will function in a particular patient. The device should not be considered a lifetime implant. 2. If the deactivation valve is closed when the cuff is inflated, fluid cannot transfer from the cuff to the balloon and sustained fecal obstruction may arise as a result: a. In the event of large pressures within the bowel, automatic pressure relief that normally occurs with the device would be prevented. Cycling the device can relieve the fecal obstruction. b. Cycling the device may be difficult if deactivation occurs when the pump bulb is deflated. If unable to cycle the prosthesis, squeezing the sides adjacent to the deactivation button will allow fluid to fill the pump bulb and then the pump can be cycled normally. c. Release of the deactivation valve may require greater pressure than that used to cycle the device. 3. Use caution when passing any instrument through the anal canal. For certain procedures, e.g. anal ultrasound or colonoscopy, first deflate the cuff then deactivate the device prior to passing any instrument through the anal canal. 4. System pressure changes may occur over time. This may result in changes in continence status. To increase system pressure, fluid may be added to the device through the septum port. 4
7 ADvERSE EvENtS No deaths, life-threatening conditions or unanticipated adverse events were reported in the clinical study that was conducted by AMS to support the safety and effectiveness of the Acticon Neosphincter. The most frequently reported device-related adverse events were pain/discomfort, infection, and erosion. Three hundred ninety-five (395) device-related or potentially device-related events occurred in 102 patients during the study. The majority (318) of the device-related adverse events were mild or moderate. Surgical interventions were required for 142 events, including 81 revisions in 56 patients. More than one type of intervention may have been used for each event and patients may have had multiple events treated with the same intervention. Table 1 lists the frequency and types of device-related adverse events reported from the clinical study and the methods of intervention used to treat the adverse events. 5
8 table 1: Methods of Intervention for Acticon Neosphincter Study Adverse Events (n=115) Methods of Intervention 3 Number and (%) Number None Adverse Event type of Patients 1 of Events 2 Required Medication Surgery other 4 Pain/Discomfort 37 (32.2) Infection 36 (31.3) Erosion 24 (20.9) Recurring fecal incontinence 22 (19.1) Constipation 22 (19.1) Impaction 21 (18.3) Surgical injury 15 (13.0) Wound problems 12 (10.4) Mechanical malfunction 12 (10.4) Wound separation 10 (8.7) Difficult evacuation 10 (8.7) Rectal bleeding 9 (7.8) Edema 9 (7.8) Erythema 9 (7.8) Fever 7 (6.1) Anorectal condition 7 (6.1) Device migration 7 (6.1) Device Fit 6 (5.2) Device Function 6 (5.2) Wound drainage 6 (5.2) Gastrointestinal condition 5 (4.3) Diarrhea 5 (4.3) Ecchymosis 4 (3.5) Malposition 4 (3.5) Device operation difficulty 3 (2.6) Other 5 27 (23.5) totals Patients may have had more than one type of event. 2 Patients may have had more than one event of the same type. 3 There may have been more than one type of intervention for each event, and patients may have multiple events that are treated with the same intervention. 4 Other interventions reported included: fluid added to pump via septum (15), enema (14), deactivation of device (13), patient education (11), and hospitalization (9). There were 18 reports of an unspecified other intervention. 5 Other adverse events included abscess (2), difficult device activation (2), hematoma (2), intraoperative bleeding (2), seroma, urinary tract infection, acute renal failure, ankle pain, chest rash, decreased perianal sensation, hip ulcer, hyperglycemia secondary to infection, mild pancreatitis, night sweats, osteomyelitis, patient dissatisfaction, patient falling, couldn t pump device, device locked, post-stoma takedown paralytic ileus, morphine reaction, respiratory distress, severe acute esophagitis, urinary retention (2), vaginal rash and weakness. The following risks of implantable anal sphincters or their materials have been reported in the medical literature but did not occur during the prospective study: tubing kink, tubing leak, cuff tab tearing, scrotal adhesion. 6
9 HoW SUPPLIED AND StoRAGE WARNING: Contents supplied STERILE. Do not use if sterile barrier is damaged. If damage is found, call your AMS representative. For single patient use only. Do not reuse, reprocess or resterilize. Reuse, reprocessing or resterilization may compromise the structural integrity of the device and/or lead to device failure which, in turn, may result in patient injury, illness or death. Reuse, reprocessing or resterilization may also create a risk of contamination of the device and/or cause patient infection or cross-infection, including, but not limited to, the transmission of infectious disease(s) from one patient to another. Contamination of the device may lead to injury, illness or death of the patient. After use, dispose of product and packaging in accordance with hospital, administrative and/or local government policy. CLINICAL StUDy A multi-center, prospective, non-randomized clinical trial was undertaken to evaluate the safety and effectiveness of the device. Each patient served as his or her own control. The primary endpoint assessed changes in patients continence using Fecal Incontinence Scoring System (FISS) scores at pre-, 6, and 12 months. Secondary endpoints assessed changes in anorectal manometry, health status, and quality of life factors affected by fecal incontinence. Self-administered questionnaires were used to assess continence, health status, and patient quality of life. Safety data related to adverse events and revision surgery were collected on case report forms. Inclusion criteria for the study included: a history of fecal incontinence for at least six months at least one prior non-surgical treatment for incontinence age > 18 years, and a FISS score of > 88. Exclusion criteria included: Crohn s Disease or Irritable Bowel Syndrome as the only cause of fecal incontinence extensive pelvic irradiation or radiation therapy that compromised the anal canal active pelvic sepsis pregnancy fragile or scarred perineum, and patients who engage in receptive anal intercourse. A total of 115 patients were enrolled at 19 sites. One-hundred twelve (112) patients were implanted with the device. Follow-up intervals occurred at activation (n=98), 6 months (n=73), and 12 months (n=69). Three-quarters of the study patients were female and the mean age was 49 (range 18-81) years. Ninety-two patients (80%) were Caucasian. Mean duration of incontinence was 14 (range 1-54) years. Table 2 presents the distribution of patients by etiology. 7
10 table 2: Distribution of Patients by Etiology Etiology of Fecal Incontinence (n=115) Frequency Percent (%) Obstetric Trauma Neurological Congenital Abnormality Anorectal Trauma Other total Patients Other etiologies included rectal prolapse (3), idiopathic (3), radiation (1), surgical (3), scleroderma (1), traumatic defecation (1), musculoskeletal (1) and anal canal squamous cell carcinoma (1) Previous treatments for fecal incontinence were separated into two categories: management and surgical treatment. Management includes less invasive forms of therapy and surgical treatment includes specific antiincontinence procedures. Several patients had previously experienced more than one type of treatment. Many patients tried and failed not only management but also surgical treatment for incontinence. Table 3 presents the distribution of patients by previous treatments. table 3: Distribution of Patients by Previous Treatments type of Previous treatment 1 Patients 1 Percent (%) Management: Bowel Management Biofeedback Surgical Treatment: Sphincteroplasty Stoma Rectal Prolapse Repair Gracilis Muscle Transposition (stimulated) Gracilis Muscle Transposition (unstimulated) Postanal Repair Other Patients with multiple types of treatments were counted more than once. Percentages were calculated for the number of patients enrolled (n=115). Patients who had more that one procedure of the same type were captured under Other. 2 Other includes: Second sphincteroplasty (10), silastic sling (3), repair of imperforate anus (4), revised colostomy (3), repeated rectal prolapse repair (2), repair neosphincter and second gracilis muscle wrap, posterior sagioplasty/vaginal anoplasty, abdominal exploratory/rectopexy and levatorplasty, posterior colporrhaphy/repair perineal body, 4-V advancement flap, neorectum, vaginal and anal reconstruction. 8
11 EFFECtIvENESS Fecal Incontinence Scoring System (FISS) The primary endpoint of the study assessed the effectiveness of the Acticon Neosphincter using the Fecal Incontinence Scoring System (FISS) patient questionnaire. The FISS instrument is a 5-item self-administered questionnaire. Scores range from and measure the patient s degree of fecal incontinence. Zero equals fully continent and 120 equals incontinent to liquid or solid stool more than once a day. Table 4 shows the FISS ranges as they relate to the level of fecal incontinence. table 4: FISS Scoring System FISS value Definition 0 Fully continent 1-30 Incontinent to gas Incontinent to seepage Incontinent to liquid or solids <monthly Incontinent to liquid or solids >monthly Incontinent to liquid or solids >weekly Incontinent to liquid or solids daily Incontinent to liquid or solids >daily Patients with a FISS score > 88 were eligible for enrollment. A FISS score of 88 or higher means the patient has incontinent episodes one or more times per week. A clinically significant improvement was demonstrated by a reduction in 12-month FISS score as compared to pre-implant FISS score of > 24 points. At the 6-month follow-up, 67 patients had both a pre-implant FISS score and a 6-month FISS score reported and thus were available for evaluation. The mean FISS scores were 106 at pre-implant and 50 at 6 months. The mean reduction in FISS score at 6 months was 56 points. Fifty-four (54) of 67 patients lowered their FISS score by 24 or more points. The success rate for the 6-month cohort was 81%. At the 12-month follow-up, 63 patients had both a pre-implant FISS score and a 12-month FISS score reported and thus were available for evaluation. The mean FISS scores were 105 at pre-implant and 48 at 12 months. The mean reduction in FISS score at 12 months was 57 points. Fifty-four (54) of 63 patients had lowered their FISS score by 24 or more points. The success rate for the 12-month cohort was 86%. Statistical analysis indicated that the differences between the mean preimplant FISS scores and the mean scores at 6 and 12 months were both statistically significant (p<0.0001). 9
12 Intent to Treat Analysis Of the 115 patients enrolled in the study, 69 (including 6 patients with preimplant stomas) reported 12-month FISS scores. Of the 46 patients not reporting 12-month FISS scores, three patients were aborted at original implant, six patients had missing data at 12-months, and 3 patients were lost to follow-up. Thirty-four patients were explanted before the 12-month follow-up. Fifty-nine (54 non-stoma and 5 stoma) patients had 24-point or greater reductions in FISS scores and were considered successes. Six patients with a pre-existing stoma completed follow-up and had 12-month FISS scores available. To be included in the intent to treat analysis, these 6 patients were assigned a pre-implant FISS score of 106, the average for the 101 non-stoma patients with pre-implant FISS scores. Using this assumption, 5 of these 6 stoma patients had a successful outcome at 12 months. By an intent to treat analysis which accounts for all enrolled patients, the clinical success rate based on 12-month FISS scores was 51.3% (59 of 115). Subgroup Analysis of Effectiveness Two way repeated measures analysis of variance (ANOVA) was performed for subgroups gender, age, etiology, and country on the primary effectiveness endpoint (FISS). The results using Two-way Repeated Measures ANOVA indicated no significant difference for the subgroups, except for gender. The analysis for FISS indicated a significant difference for males and females. The statistical difference in FISS for males and females does not appear to have clinical implications due to the observed reduction in FISS at 12 months (male=50, female=58). The reductions are greater than the 24-point reduction that defines clinical success in the study protocol. Males demonstrated a 42-point (6 months) and 50-point drop (12 months) in FISS scores. Females demonstrated a 59-point (6 months) and 58-point drop (12 months) in FISS scores. Anorectal Manometry Anorectal manometry was used to assess anal sphincter function. Patients were assessed by analyzing the difference in manometric resting pressures between the pre-implant visit and follow-up intervals. 10
13 table 5: Anorectal Manometry Resting Pressures (mmhg) 6-Month 12-Month Pre-Implant Activation Post-Activation Post-Activation Mean Minimum Maximum Standard Deviation n Average resting pressures increased from 26 mmhg pre-implant to 45 mmhg at 12 months (n=53). The increase in resting pressures from pre-implant to 12 months was significant (p<0.0001). A correlation between higher resting pressures and lower FISS scores was indicated at 12 months (correlation = -0.38; p=0.0039). Health Status Questionnaire (HSQ) The Health Status Questionnaire, developed by Health Outcomes Institute, is a 39-item self-administered questionnaire assessing the patient s own health perceptions and the impact of health on physical functioning, social functioning, and mental health. The HSQ was developed from the SF-36 and MOS-20 questionnaires. The scale for each domain ranges from with 100 representing ideal functioning. The total HSQ score is obtained by adding the score from each of the eight domains. table 6: Health Status Questionnaire Results Mean D pre-implant Scale to 12-months (n=48) p-value Health Perception Physical Functioning < Role Limitations/Physical Health Role Limitations/Emotional Problems Social Functioning < Mental Health Bodily Pain Energy/Fatigue Overall HSQ < Data was available from 48 patients at 12-month follow-up. HSQ mean scores from pre-implant and 12-month follow-up were analyzed and compared. The change in score was significant if the p-value < Table 6 lists the scales, the change in scores, and whether or not a significant change was observed. All scales except the Role Limitations/Emotional Problems and the Bodily Pain scales indicated a significant improvement from pre-implant to the 12-month visit. While these two scales did not show significance, they did 11
14 indicate a change in the positive direction. Overall, the HSQ results indicated a significant improvement in status from pre-implant to 12-month visit. Fecal Incontinence Quality of Life The Fecal Incontinence Quality of Life Questionnaire (FIQOL) is a 29-item, self-administered questionnaire designed to assess the impact of fecal incontinence on a variety of activities and feelings. It is a psychometric evaluation, which objectively measures the physical, psychological, and social impact that fecal incontinence has on the patient s lifestyle. Pre-implant results of the FIQOL demonstrated the distressing effects of fecal incontinence on study patients. The decreases in FIQOL response percentages indicated that the physical, psychological, and social impacts that fecal incontinence had on the patients lifestyle were diminished from pre-implant to the 12-month follow-up. table 7: Fecal Incontinence Quality of Life Pre-implant 12-months Characteristic (n=113) (n=67) Feel I have no control over bowels 89% 9% Worry about bowel accidents* 86% 22% Alter activities to be near bathroom 81% 33% Worry about being embarrassed* 80% 25% Bowel accidents always on mind* 78% 25% Use pads 77% 39% Worry about others smelling stool on me* 76% 19% Locate bathrooms when someplace new 76% 34% My life is more difficult 70% 15% Avoid wearing light colored clothing 69% 24% Cannot do the things I want to do* 68% 16% Feel ashamed* 66% 18% Plan schedule around bowels 65% 21% Table 7 lists patient responses to statements about fecal incontinence and its effects. The percentages indicate responses of either Most of the time or Strongly agree. For example, when asked to respond to the statement Due to accidental bowel leakage, I feel I have no control over my bowels, 89% of patients responded Most of the time before implant whereas 9% responded Most of the time at 12 months. The response choices were None, A little, Some, or Most of the time. Patients responded Strongly agree to statements marked with an asterisk (*). Response choices were Strongly disagree, Somewhat disagree, Somewhat agree, and Strongly agree. 12
15 REvISIoN SURGERy Revisions were reported in the clinical trial. Revision surgery included repositioning, removing and/or replacing one or more device components. The 395 device-related adverse events in the study resulted in 81 device revisions. The 81 revisions occurred in 56 out of 112 implanted patients. The patient revision rate for the study was 50.0%. No long-term adverse sequelae were reported in association with revision surgery in the clinical trial. table 8: Device Revisions from Acticon Neosphincter Clinical Study % of Number of Number of patients Reasons for revision 1 Events 2 Patients (n=112) Infection Erosion Malfunction Recurring Incontinence Migration Pain Patient Dissatisfaction Malposition Other total: More than one reason may exist for each revision e.g., infection and erosion. 2 Patients may have multiple occurrences of the same event type. 3 Other reasons for revision included replacing previously removed cuff (2), incision open (1), two stage device removal (2), cuff opened (3), cellulitis/erosion of the peritoneal skin (1), improperly sized cuff (1), constipation (2) and ano-urethral communication (1) The most common indications for revision surgery were infection (28 patients) and erosion (24 patients). Overlap of infection and erosion occurred in 13 cases. Cuff erosions occurred in 22 patients (rectum 12, perineum 10). Pump erosions occurred in four (4) patients and one (1) patient had a tubing erosion. Some patients experienced more than one type of erosion. The third most frequent cause for revision was device malfunction. The revision rate for malfunctions was 9.8%. Fourteen patients (14) had two revisions, one patient had three revisions, and three patients had four revisions. Ten patients who underwent one or more revision surgeries prior to the 12-month visit had functional devices at 12 months. Six of these patients were considered as clinical successes at 12 months. Two additional patients were stoma patients who would be considered as clinical successes if assigned the average pre-implant FISS score of
16 Device Explants Device explants represent a subset of the 81 revisions listed in Table 8. The reasons for device explant are listed in Table 9. At the 12-month follow-up, 34 patients (30% of implanted patients) experienced 38 explants. The mean time from implant to explant was 4.2 months ( ). table 9: Device Explants from Acticon Neosphincter Clinical Study Reasons for explant Number of Events 1 Infection 12 Infection and erosion 12 Erosion 11 Recurring Incontinence and pain 2 Ano-urethral communication 1 total Explants 38 1 Patients may have multiple occurrences of the same event type. Infection, erosion, or a combination of infection and erosion accounted for 35 of the 38 explants. Out of the 34 patients who had explants, 7 are candidates for reimplant and 27 are permanent explants. The 27 permanent explants exited the study. Use of a Standard Prophylactic Antibiotic Regimen (SPAR) During the clinical study, an infectious disease specialist, in conjunction with AMS, determined that the infections associated with the device demonstrated a much broader array of microorganisms than was noted with other types of implantable device surgery. To provide the broader coverage needed for these cutaneous and bowel microorganisms he recommended a different combination of antibiotics than normally used. AMS sent this recommendation out to all clinical sites and 16 of the 112 patients were implanted using a recommended standard prophylactic antibiotic regimen (SPAR 1), consisting of one of the following: a. Piperacillin-Tazobactam (Zosyn) 3.375gm IV preop, then Q6h x 3 doses postop. Plus, Vancomycin 1gm IV preop once only. b. For patients allergic to penicillins: Cefotetan 2gm IV preop and Q12h x 2 doses postop. Plus, Vancomycin 1gm IV preop. c. For patients allerigic to penicillins and cephalosporins: Trovofloxacin 300mg IV preop and possibly Q24h x 1 dose. Plus, Vancomycin 1 gm IV preop. d. For patients allerigic to penicillins and cephalosporins and vancomycin: Trovofloxacin 300mg IV preop and possibly Q24h x 1 dose. 14
17 Only two of the 16 patients treated with this SPAR 1 (12.5%) developed an infection leading to revision or explant during the clinical study. A separate, follow-up study was conducted involving 25 patients using a different SPAR (SPAR 2) that consisted of one of the following: a. Cefotetan 2 gm IV + Vancomycin 1 gm IV x 1 preop b. For patients allergic to vancomycin: Cefotetan 2 gm IV + Linezolid 600 mg IV x 1 preop c. For patients allergic to cephalosporins or a history of anaphylactic sensitivity to penicillin and no history of tolerating a prior cephalosporin: Vancomycin 1 gm IV + Cipro 400 mg IV + Flagyl 500 mg IV x 1 preop Timing of the prophylaxis is very important: the infusion was to be completed 0 60 minutes before the first incision. The results from this study using are presented in Table 10. table 10: Incidence of Infection Among Patients Treated with SPAR 2 Patients enrolled, N 25 Patients with implanted devices, 1 N 22 Patient lost to follow-up 2 1 Infection incidence for remaining 21 patients 21 N patients who developed infection 3 2 (9.5%) N patients without infection 19 (90.5%) 1 Three patients were withdrawn from the study at time of implant. 2 One patient was lost to follow-up 15 days after implant. 3 Two patients had their devices explanted 11 and 49 days after implant due to infection. Because one patient treated with SPAR 2 was followed for only 148 days (5 months), when that same time frame is applied to SPAR 1 patients, only 1/16 (6.3%) experienced an infection leading to revision or explant < 5 months after implant. Combining patients treated with both SPAR 1 and SPAR 2, 8.1% (3/37 [1/38 was lost to follow-up 15 days after implant]) experienced an infection leading to explant or revision < 5 months of implant. In contrast, when the 16/112 patients with SPAR 1 are removed, 21.9% (21) of the remaining 96 patients in the original clinical study experienced an infection leading to explant or revision over that same 5 month time period. PAtIENt CoUNSELING INFoRMAtIoN Patients should be counseled in order to have a realistic expectation of the physical, psychological and functional outcome of the implantation. The risks, benefits and potential adverse events of all available treatment options should be discussed with the patient and considered by the physician and patient when choosing a treatment option. Patient information brochures are 15
18 available from AMS to help the patient understand these issues. The brochures also discuss the device, the implant procedure, how-to-use the device, and results from the clinical study. The patient information brochures should be provided to patients prior to the surgery. Some patients may become dissatisfied by the presence of the prosthetic device in their body. This issue should be discussed with the patient prior to the surgery. Patient dissatisfaction may lead to device removal. Patients should also be aware that the Acticon Neosphincter is not considered a lifetime implant. An appropriate patient history, including history of personality disorders, and diagnostic work-up should be a part of the patient decision making process. Discuss with the patient the possibility of an allergic reaction to the materials in the device (See Silicone Information). SILICoNE INFoRMAtIoN This device is composed of a number of materials, including solid silicone elastomers and a fluorosilicone lubricant. Silicone gel is not a component in the materials of this device. Solid silicone elastomers have been commonly used in a variety of biomedical devices for over 40 years. Silicone fluids have an extensive history of use in medical devices. Scientific literature has included reports of adverse events and other observations in patients with implantable silicone devices. As reported, these events/observations indicate allergic-like symptoms and in other cases a symptom complex associated with immunological disorders. No casual relationship has been established between these events and silicone elastomer or fluorosilicone lubricant. There are reports of malignant tumor formation in laboratory animals only associated with implants of relatively large size. Many different materials are associated with this effect in animals, silicone elastomers among them. No such effect has been described in humans. Extensive testing has been conducted on all materials in the AMS Acticon Neosphincter. This testing indicated that no toxicological response was attributable to the materials. However, some of the materials caused minor irritation when implanted in animals. Silicone elastomer particulate shedding and particulate migrations to regional lymph nodes have been reported in the literature on penile implants. There are no known clinical sequelae to this phenomenon. MAGNEtIC RESoNANCE IMAGING (MRI) INFoRMAtIoN Several studies regarding MRI and AMS prostheses have concluded that the presence of an AMS prosthesis will not produce harmful effects during scanning. These studies were conducted by Robert C. Lange, Ph.D., Yale University and Frank G. Shellock, Ph.D., Cedars-Sinai Medical Center, Los Angeles. Dr. Lange produced his study for American Medical Systems 16
19 and Dr. Shellock produced his studies independently for publication in the 1, 2, 3, 4 American Journal of Roentgenology (AJR) and Radiology. In these studies, the metallic components in AMS prostheses were subjected to magnetic field strengths up to 1.5 Tesla and showed no unsafe magnetic interaction. The small stainless steel components in AMS prostheses may distort the uniform magnetic field in the vicinity of the implant, although it is unlikely that these components will interfere with normal MRI. However, the complete compatibility profile of these products within a MRI field has not been established. INvENtoRy REtURNS AND PRoDUCt REPLACEMENt INFoRMAtIoN Before returning any components, whether explanted or unused (sterile or nonsterile), customers must fill out the Return Goods Form located on the last page of the Patient Information Form. Follow all of the instructions on the form carefully, and be sure that the components have been thoroughly cleaned before returning them to AMS. In all cases, obtaining credit or percentage of credit for a returned component is subject to approval under the terms of the AMS Return Goods Policy and the AMS Product Replacement Policy. For complete information regarding these policies, contact the AMS Customer Service Department. 1 Shellock F, MR Imaging of Metallic Implants and Materials: A Compilation of the Literature, AJR, October Shellock F, MR Imaging and Biomedical Implants, Materials and Devices: An Updated Review, Radiology, 1991, Vol 180, pp Shellock F, MR Procedures and Biomedical Implants, Materials and Devices: 1993 Update, Radiology, 1993, Vol. 189, pp Shellock F, MR Procedures and Metallic Objects: Update Philadelphia, Lippincott-Raven, 1997, pp. 101,
20
21 Français Mode d emploi Néosphincter Acticon AMS REMARQUE : Pour plus de renseignements sur le néosphincter Acticon et son implantation, se reporter au manuel opératoire. BRèvE DESCRIPtIoN DE LA PRotHèSE Le néosphincter Acticon est un dispositif implantable en élastomère de silicone, rempli de liquide, destiné à traiter l incontinence fécale grave. L incontinence fécale grave se définit comme la perte involontaire de selles aqueuses ou solides plus d une fois par semaine. Le néosphincter Acticon peut être implanté chez l homme ou la femme et il se compose de trois composants interconnectés : une manchette occlusive, un ballon régulateur de pression et une pompe de contrôle munie d un septum. Ces trois éléments sont reliés entre eux par une tubulure résistant aux plicatures. Le néosphincter Acticon simule la fonction normale du sphincter de l anus en permettant l ouverture du canal anal selon la volonté du patient. La manchette occlusive est implantée autour d un segment du canal anal. La prothèse assure la continence du patient à l aide de la pression de la manchette remplie de liquide qui permet l occlusion du canal anal. Pour l évacuation intestinale, le patient comprime et relâche plusieurs fois le mécanisme de la pompe située dans la lèvre ou le scrotum, afin de transférer le liquide de la manchette au ballon régulateur de pression implanté dans l espace prévésical. Ce déplacement de liquide vide et entraîne l affaissement de la manchette, relâchant ainsi la force de compression exercée autour du canal anal. La pression résiduelle à l intérieur du ballon permet le retour du liquide dans la manchette qui se remplit auto - matiquement en l espace de quelques minutes. Le ballon régulateur de pression maintient la pression à l intérieur de la manchette occlusive. Cet implant contient de l élastomère de silicone. INDICAtIoN Le néosphincter Acticon est un implant utilisé pour le traitement de l inconti - nence fécale grave chez l homme et la femme âgé(e) de dix-huit ans et plus pour qui des formes moins invasives de restauration de la fonction ano-rectale n ont donné aucun résultat ou sont déconseillées. 19
22 CoNtRE-INDICAtIoNS 1. La mise en place de cet implant est contre-indiquée chez les patients jugés par le médecin comme des candidats peu susceptibles d en bénéficier, soit parce qu une opération est risquée, soit à cause de leurs antécédents médicaux (condition physique ou mentale). 2. Cet implant est contre-indiqué chez les patients souffrant d incontinence fécale compliquée par l obstruction irréversible d un segment proximal de l intestin. 3. L usage de cet implant est contre-indiqué chez les patients souffrant d une infection évolutive. AvERtISSEMENtS 1. Les patients souffrant de diabète, de lésions de la moelle épinière, de difformités musculo-squelettiques, de stomie antérieure ou de plaies ouvertes dans le site opératoire présentent un risque accru d infection liée à une prothèse. Les patients qui sont immunodéprimés ou victimes d immunosuppression peuvent aussi présenter un plus grand risque d infection liée à une prothèse. Des mesures appropriées doivent être prises afin de réduire ce risque. Une infection ne répondant pas au traitement antibiotique peut nécessiter le retrait de l implant. Une infection suivie d une explantation peut causer des cicatrices qui risquent de rendre la réimplantation plus difficile. 2. Une érosion peut survenir suite à une infection, une pression exercée sur les tissus, une manchette de taille incorrecte, un ballon inadapté, l endommagement des tissus ou le positionnement incorrect d un élément de l implant. Il peut se produire une érosion de la manchette autour du canal anal ou à travers la peau périnéale. Une érosion de la pompe de contrôle peut survenir à travers la peau du scrotum ou des lèvres. Le ballon régulateur de pression peut exercer une érosion sur la vessie. Une érosion qui n est pas évaluée et traitée rapidement peut entraîner une forte détérioration de l état du patient, avec infection et perte de tissus. 3. Cet implant contient des élastomères de silicone. Le gel de silicone n entre pas dans sa composition. Il est conseillé d évaluer avec soin les avantages et les inconvénients de son implantation chez les patients présentant une sensibilité connue au silicone. 4. Les complications chirurgicales, physiques, psychologiques ou mécaniques peuvent exiger une intervention corrective ou le retrait de l implant. Le retrait sans réimplantation rapide d un nouveau dispositif peut compliquer, voire rendre impossible, une réimplantation ultérieure. Le moment de la réimplantation doit être choisi par le médecin traitant en fonction de l état pathologique et des antécédents médicaux du patient. 20
23 PRéCAUtIoNS Patient 1. Une consultation et une évaluation préopératoires par le chirurgien s imposent pour une bonne sélection des patients. 2. Le patient devra être préparé à avoir des attentes réalistes quant aux résultats physiques, psychologiques et fonctionnels de l implantation d un néosphincter Acticon. Même si cet implant est destiné à redonner aux patients le contrôle de leurs intestins, certains d entre eux continuent à souffrir d incontinence après l implantation. 3. Certains patients peuvent éprouver des douleurs lors de la mise en service de l implant au cours de la période postopératoire et pendant les premières utilisations de l implant. Quelques cas de douleur chronique due à l implan - tation ont été signalés. Des douleurs anormalement intenses ou prolongées pourraient exiger une intervention médicale ou chirurgicale. Il convient d avertir le patient de l éventualité d une douleur postopératoire, y compris son intensité et sa durée. 4. Une fibrose des tissus ou des antécédents chirurgicaux dans la zone d implantation peuvent rendre impossible l implantation d une manchette occlusive au niveau du canal anal. 5. Une maladie intestinale aiguë, par ex. une diarrhée ou une constipation, peut nuire au bon fonctionnement de l implant et nécessiter l emploi de garnitures externes ou de manipulations pour faciliter la défécation. 6. Une maladie dégénérescente progressive, telle que la sclérose en plaques, peut limiter l utilité future de l implant en tant que traitement de l inconti - nence fécale. 7. L utilisation de l implant nécessite de la part du patient une dextérité manuelle, une force et une motivation adéquates. 8. Un traumatisme de la région pelvienne, périnéale ou abdominale, par exemple une chute lors d une activité sportive, peut endommager l implant et les tissus environnants. Il peut en outre entraîner la défaillance de l implant et nécessiter une intervention corrective, voire le remplacement de l implant. Il incombe au médecin d avertir ses patients de ces possibilités et de leur conseiller d éviter tout traumatisme de ces régions. 9. Si l implant est rempli d une solution radio-opaque au lieu d une solution saline isotonique stérile, s assurer que le patient ne souffre pas d allergie à la solution radio-opaque. 10. Des relations sexuelles anales passives peuvent endommager la manchette occlusive et sont déconseillées aux patients porteurs de cette prothèse. 11. L accouchement par la voie basse peut nuire au fonctionnement ultérieur de la manchette occlusive. Les patientes doivent être conscientes du besoin de discuter de la présence de l implant Acticon avec leur médecin en cas de grossesse. Un accouchement par césarienne peut être recommandé afin d éviter d endommager l implant. 21
24 12. Il n existe aucune donnée en matière d innocuité ou d efficacité pour les patients ayant des antécédents de maladie intestinale inflammatoire ou de rayonnement du bassin. La possibilité d un risque d érosion accru chez ces patients n est pas connue. Chirurgie 1. L implantation d une manchette de taille incorrecte, d un ballon inadapté ou d autres facteurs, tels qu un traumatisme chirurgical, une mauvaise visibilité des tissus et des procédures médicales associées, peuvent causer une érosion des tissus, une migration des éléments de l implant ou la persistance de l incontinence. 2. La migration des éléments de l implant est possible si la taille de la man - chette, la position de la pompe ou du ballon, ou la longueur de la tubulure sont incorrectes. Elle peut entraîner des douleurs, des complications, la défaillance de l implant et une révision chirurgicale. 3. Une technique chirurgicale incorrecte, une mise en place anatomique erronée des éléments de l implant, une manchette de taille inadaptée et/ou un liquide de remplissage inapproprié sont susceptibles de compromettre le succès de l implantation. 4. Bien que la tubulure renforcée ait été conçue pour être plus résistante aux plicatures, des plicatures peuvent tout de même se produire si la longueur de la tubulure implantée est inadéquate. Implant 1. Cet implant s use à l usage et peut avoir une défaillance après un certain temps. Il est impossible de prévoir la durée de service d un implant chez un patient donné. Il ne s agit pas d un implant à vie. 2. Si la soupape de désactivation est fermée lors du gonflement de la manchette, le liquide ne peut pas passer de la manchette au ballon, ce qui peut entraîner une obstruction prolongée des matières fécales. a. En cas de fortes pressions dans les intestins, le relâchement automatique de la pression qui survient normalement avec ces implants ne se produira pas. La réactivation de l implant pour un cycle complet peut soulager l obstruction des matières fécales. b. La réactivation de l implant pour un cycle complet peut être difficile si la désactivation se produit quand la pompe est dégonflée. Dans ce cas, comprimer les côtés adjacents au bouton de désactivation afin de remplir la pompe pour pouvoir mettre l implant en service. c. Le délogement de la soupape de désactivation peut exiger une pression plus forte que celle nécessaire pour l activation de l implant. 3. Faire attention lors du passage d instrument à travers le canal anal. Pour certaines procédures, par ex., une échographie anale ou une côlonoscopie, dégonfler d abord la manchette, puis désactiver la prothèse avant d introduire un instrument dans le canal anal. 22
25 4. Des changements de pression peuvent survenir dans le système après un certain temps. Ils peuvent causer des modifications de l état de la continence. Pour augmenter la pression du système, il est possible d ajouter du liquide à la prothèse par le port du septum. CoMPLICAtIoNS Aucun décès, affection mettant en jeu le pronostic vital ou réactions indé - sirables inattendues n ont été signalés dans l étude clinique menée par AMS pour démontrer l innocuité et l efficacité du néosphincter Acticon. Les effets indésirables liés à l implant le plus souvent rapportés étaient la sensation de douleurs ou de gêne, l infection et l érosion. Trois cent quatre-vingt-quinze (395) complications liées ou susceptibles d être liées à l implant sont survenues chez 102 patients au cours de l étude. La majorité (318) d entre elles étaient bénignes ou modérées. Une inter - vention chirurgicale s est avérée nécessaire pour 142 complications, dont 81 révisions chez 56 patients. Plus d un type d intervention a pu être utilisé pour chaque complication et des patients ont pu faire traiter plusieurs complications au cours d une même intervention. Le tableau 1 indique la fréquence et les types de complication liée à l implant qui ont été rapportés par l étude clinique, ainsi que les méthodes d intervention suivies pour les traiter. 23
26 tableau 1 : Méthodes d intervention pour les complications relevées dans l étude sur le néosphincter Acticon (n=115) Méthodes d intervention 3 Nombre Nombre et (%) de compli- Aucune type de complication de patients 1 cations 2 nécessaire Médication Chirurgie Autre 4 Douleur/gêne 37 (32.2) Infection 36 (31.3) Érosion 24 (20.9) Incontinence fécale récidivante 22 (19.1) Constipation 22 (19.1) Fécalome 21 (18.3) Traumatisme chirurgical 15 (13.0) Problèmes de cicatrisation 12 (10.4) Défaillance mécanique 12 (10.4) Ouverture de la plaie 10 (8.7) Difficultés lors de l évacuation 10 (8.7) Saignement rectal 9 (7.8) Œdème 9 (7.8) Erythème 9 (7.8) Fièvre 7 (6.1) État ano-rectal 7 (6.1) Migration de l implant 7 (6.1) Ajustement de l implant 6 (5.2) Fonctionnement de l implant 6 (5.2) Drainage de la plaie 6 (5.2) État gastro-intestinal 5 (4.3) Diarrhée 5 (4.3) Ecchymose 4 (3.5) Mauvaise position 4 (3.5) Fonctionnement difficile de l implant 3 (2.6) Autre 5 27 (23.5) totaux Un même patient peut avoir eu plusieurs types de complication. 2 Un même patient peut avoir eu plusieurs complications du même type. 3 Il peut y avoir eu plusieurs types d intervention pour chaque complication et un même patient peut avoir eu plusieurs complications qui ont été traitées par la même intervention. 4 Les autres interventions signalées comprennent : ajout de liquide à la pompe par le septum (15), lavement (14), désactivation de l implant (13), éducation du patient (11) et hospitalisation (9). Il y a eu 18 rapports d intervention «Autre» non spécifiée. 5 Les autres complications signalées comprennent : abcès (2), activation difficile de l implant (2), hématome (2), saignement peropératoire (2), sérome, infection des voies urinaires, insuffisance rénale aiguë, douleur à la cheville, éruption cutanée sur la poitrine, perte de sensation périanale, ulcère de hanche, hyperglycémie consécutive à une infection, pancréatite bénigne, sueurs nocturnes, ostéomyélite, insatisfaction du patient, chute du patient, pompage impossible de la prothèse, prothèse bloquée, iléus paralytique après démontage post-stomie, réaction d hypersensibilité à la morphine, détresse respiratoire, grave œsophagite aiguë, rétention urinaire (2), éruption cutanée vaginale et faiblesse. Les complications suivantes présentées par les sphincters anaux implantables ou leurs matériaux figurent dans les publications médicales mais ne se sont pas produites au cours de l étude prospective : plicatures de tubulure, fuite de tubulure, déchirure de languette de manchette, adhérence au scrotum. 24
Kegel exercises spanish pdf
 Kegel exercises spanish pdf not do this as an exercise on the toilet. HEALTH EDUCATION. Kegels: Pelvic Floor Strengthening. Exercises for Women. What are pelvic floor muscles? 30 Jun 2017. Los músculos
Kegel exercises spanish pdf not do this as an exercise on the toilet. HEALTH EDUCATION. Kegels: Pelvic Floor Strengthening. Exercises for Women. What are pelvic floor muscles? 30 Jun 2017. Los músculos
TSQM (Version 1.4) Treatment Satisfaction Questionnaire for Medication
 TSQM (Version 1.4) Treatment Satisfaction Questionnaire for Medication Instructions: Please take some time to think about your level of satisfaction or dissatisfaction with the medication you are taking
TSQM (Version 1.4) Treatment Satisfaction Questionnaire for Medication Instructions: Please take some time to think about your level of satisfaction or dissatisfaction with the medication you are taking
Thank you. US English US Spanish. Australia-English Canada-English Ireland-English New Zealand-English Taiwan-English United Kingdom-English
 Dear Healthcare Provider, Included in this PDF are recruitment brochures in several languages to be used in MM Bone study (Protocol No.: 20090482). Kindly note these brochures have been updated according
Dear Healthcare Provider, Included in this PDF are recruitment brochures in several languages to be used in MM Bone study (Protocol No.: 20090482). Kindly note these brochures have been updated according
EL IMPACTO DEL DOLOR: DATOS EUROPEOS Y ESPAÑOLES
 EL IMPACTO DEL DOLOR: DATOS EUROPEOS Y ESPAÑOLES César Margarit Unidad del Dolor Hospital General de Alicante Member of the Change Pain International Advisory Board PARA PRODUCIR UN CAMBIO NATIONAL HEALTH
EL IMPACTO DEL DOLOR: DATOS EUROPEOS Y ESPAÑOLES César Margarit Unidad del Dolor Hospital General de Alicante Member of the Change Pain International Advisory Board PARA PRODUCIR UN CAMBIO NATIONAL HEALTH
The 10 Building Blocks of Primary Care
 The 10 Building Blocks of Primary Care My Action Plan Background and Description The Action Plan is a tool used to engage patients in behavior-change discussion with a clinician or health coach. Using
The 10 Building Blocks of Primary Care My Action Plan Background and Description The Action Plan is a tool used to engage patients in behavior-change discussion with a clinician or health coach. Using
Types of Dementia. Common Causes of Dementia
 Types of Dementia Dementia is a loss of skills to think, remember and reason that is severe enough to affect daily activities. It is normal to need more time to remember things as we get older. Other thinking
Types of Dementia Dementia is a loss of skills to think, remember and reason that is severe enough to affect daily activities. It is normal to need more time to remember things as we get older. Other thinking
IE12_ CONSOLIDACIÓN Y DESARROLLO DE NUEVAS TÉCNICAS DE EVALUACIÓN INTENSIVAS ON-LINE YA IMPLEMENTADAS POR EL GIE E4
 IE12_13-03001 - CONSOLIDACIÓN Y DESARROLLO DE NUEVAS TÉCNICAS DE EVALUACIÓN Departamento de Estructuras de la Edificación Escuela Técnica Superior de Arquitectura de Madrid Universidad Politécnica de Madrid
IE12_13-03001 - CONSOLIDACIÓN Y DESARROLLO DE NUEVAS TÉCNICAS DE EVALUACIÓN Departamento de Estructuras de la Edificación Escuela Técnica Superior de Arquitectura de Madrid Universidad Politécnica de Madrid
Matemáticas Muestra Cuadernillo de Examen
 Matemáticas Muestra Cuadernillo de Examen Papel-Lápiz Formato Estudiante Español Versión, Grados 3-5 Mathematics Sample Test Booklet Paper-Pencil Format Student Spanish Version, Grades 3 5 Este cuadernillo
Matemáticas Muestra Cuadernillo de Examen Papel-Lápiz Formato Estudiante Español Versión, Grados 3-5 Mathematics Sample Test Booklet Paper-Pencil Format Student Spanish Version, Grades 3 5 Este cuadernillo
Volatilidad: Noviembre 2010 Futuros Frijol de Soya
 Observaciones Junio 09, 2010 1. La volatilidad tiene una tendencia a aumentar de Junio a Julio. 2. Este reporte sugiere que se debería considerar la implementación de estrategias largas con opciones en
Observaciones Junio 09, 2010 1. La volatilidad tiene una tendencia a aumentar de Junio a Julio. 2. Este reporte sugiere que se debería considerar la implementación de estrategias largas con opciones en
Male Organ Dysfunction 101: Symptoms, Causes, and Treatment
 Male Organ Dysfunction 101: Symptoms, Causes, and Treatment EGuys may do a lot of talking about sensual conquests and the unrelenting prowess of their libido; however, one sensual thing a lot of men won
Male Organ Dysfunction 101: Symptoms, Causes, and Treatment EGuys may do a lot of talking about sensual conquests and the unrelenting prowess of their libido; however, one sensual thing a lot of men won
Workers Compensation Non-Subscriber Form
 Workers Compensation Non-Subscriber Form Texas is unique in one very important respect: It s the only state in which employers have the choice to carry workers compensation insurance or not. There are
Workers Compensation Non-Subscriber Form Texas is unique in one very important respect: It s the only state in which employers have the choice to carry workers compensation insurance or not. There are
iclef-2002 at Universities of Alicante and Jaen University of Alicante (Spain)
 iclef-2002 at Universities of Alicante and Jaen University of Alicante (Spain) ! Introduction! Passage Retrieval Systems! IR-n system! IR-n system at iclef-2002! Conclusions and Future works ! Introduction!
iclef-2002 at Universities of Alicante and Jaen University of Alicante (Spain) ! Introduction! Passage Retrieval Systems! IR-n system! IR-n system at iclef-2002! Conclusions and Future works ! Introduction!
Bystander effect. Amy Lozano White Radiology and physical medicine Year 4 of Medicine Studies
 Bystander effect Amy Lozano White Radiology and physical medicine Year 4 of Medicine Studies Key points Concept Historical evolution Paradigm change Mechanisms Its implications in radiological protection
Bystander effect Amy Lozano White Radiology and physical medicine Year 4 of Medicine Studies Key points Concept Historical evolution Paradigm change Mechanisms Its implications in radiological protection
Personal Dosimetry Statistics and Specifics of Low Dose Evaluation
 Personal Dosimetry Statistics and Specifics of Low Dose Evaluation Comisión Chilena de Energía Nuclear Ricardo Avila, Roberto Gómez, Personal Dosimetry Laboratory and Carlos Oyarzún, Metrology of Ionizing
Personal Dosimetry Statistics and Specifics of Low Dose Evaluation Comisión Chilena de Energía Nuclear Ricardo Avila, Roberto Gómez, Personal Dosimetry Laboratory and Carlos Oyarzún, Metrology of Ionizing
Learning Masters. Early: Force and Motion
 Learning Masters Early: Force and Motion WhatILearned What important things did you learn in this theme? I learned that I learned that I learned that 22 Force and Motion Learning Masters How I Learned
Learning Masters Early: Force and Motion WhatILearned What important things did you learn in this theme? I learned that I learned that I learned that 22 Force and Motion Learning Masters How I Learned
Brain Tumors. Glioma Tumors
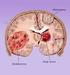 Brain Tumors A tumor that starts and grows in the brain is called a primary brain tumor. Primary brain tumors get their name from the type of cell or the part of the brain where they start growing. A secondary
Brain Tumors A tumor that starts and grows in the brain is called a primary brain tumor. Primary brain tumors get their name from the type of cell or the part of the brain where they start growing. A secondary
AMS 800 Urinary Control System For Female and Pediatric Patients. AMS 800 Système de contrôle urinaire pour les femmes et les enfants
 AMS 800 Urinary Control System For Female and Pediatric Patients Instructions for Use English Français Deutsch Italiano Español Português AMS 800 Urinary Control System For Female and Pediatric Patients
AMS 800 Urinary Control System For Female and Pediatric Patients Instructions for Use English Français Deutsch Italiano Español Português AMS 800 Urinary Control System For Female and Pediatric Patients
MANUAL EASYCHAIR. A) Ingresar su nombre de usuario y password, si ya tiene una cuenta registrada Ó
 MANUAL EASYCHAIR La URL para enviar su propuesta a la convocatoria es: https://easychair.org/conferences/?conf=genconciencia2015 Donde aparece la siguiente pantalla: Se encuentran dos opciones: A) Ingresar
MANUAL EASYCHAIR La URL para enviar su propuesta a la convocatoria es: https://easychair.org/conferences/?conf=genconciencia2015 Donde aparece la siguiente pantalla: Se encuentran dos opciones: A) Ingresar
Sistemas de impresión y tamaños mínimos Printing Systems and minimum sizes
 Sistemas de impresión y tamaños mínimos Printing Systems and minimum sizes Para la reproducción del Logotipo, deberán seguirse los lineamientos que se presentan a continuación y que servirán como guía
Sistemas de impresión y tamaños mínimos Printing Systems and minimum sizes Para la reproducción del Logotipo, deberán seguirse los lineamientos que se presentan a continuación y que servirán como guía
PROBLEMAS PARA LA CLASE DEL 20 DE FEBRERO DEL 2008
 PROBLEMAS PARA LA CLASE DEL 20 DE FEBRERO DEL 2008 Problema 1 Marketing estimates that a new instrument for the analysis of soil samples will be very successful, moderately successful, or unsuccessful,
PROBLEMAS PARA LA CLASE DEL 20 DE FEBRERO DEL 2008 Problema 1 Marketing estimates that a new instrument for the analysis of soil samples will be very successful, moderately successful, or unsuccessful,
Save Money 2-up Single Doorhanger Set OH payday advance edition, 4 different doorhangers, Spanish
 Save Money 2-up Single Doorhanger Set OH payday advance edition, 4 different doorhangers, Spanish PACKAGE CONTENTS How to Customize 4-color doorhanger, Editable PDF (50% OFF first loan) 1-color (black)
Save Money 2-up Single Doorhanger Set OH payday advance edition, 4 different doorhangers, Spanish PACKAGE CONTENTS How to Customize 4-color doorhanger, Editable PDF (50% OFF first loan) 1-color (black)
Going Home. Medicines. Pain. Diet
 Going Home After an illness or injury, some things may change in your life. Make sure you and your family know the answers to these questions before you go home from the hospital. Medicines Am I taking
Going Home After an illness or injury, some things may change in your life. Make sure you and your family know the answers to these questions before you go home from the hospital. Medicines Am I taking
TOUCH MATH. Students will only use Touch Math on math facts that are not memorized.
 TOUCH MATH What is it and why is my child learning this? Memorizing math facts is an important skill for students to learn. Some students have difficulty memorizing these facts, even though they are doing
TOUCH MATH What is it and why is my child learning this? Memorizing math facts is an important skill for students to learn. Some students have difficulty memorizing these facts, even though they are doing
Final Project (academic investigation)
 Final Project (academic investigation) MÁSTER UNIVERSITARIO EN BANCA Y FINANZAS (Finance & Banking) Universidad de Alcalá Curso Académico 2015/16 GUÍA DOCENTE Nombre de la asignatura: Final Project (academic
Final Project (academic investigation) MÁSTER UNIVERSITARIO EN BANCA Y FINANZAS (Finance & Banking) Universidad de Alcalá Curso Académico 2015/16 GUÍA DOCENTE Nombre de la asignatura: Final Project (academic
For more information regarding these forms please go to the Texas Department of Insurance website http://www.tdi.state.tx.us/forms/form20employer.
 CAPROCK Claims Management, LLC ROCK SOLID PERFORMANCE AND RESULTS PO Box 743427 Dallas, TX 75374 (888) 812-3577 Fax (972) 934-3091 IMPORTANT NOTICE FOR REQUIRED FILING FORMS DWC FORM-5 & DWC FORM-7 Caprock
CAPROCK Claims Management, LLC ROCK SOLID PERFORMANCE AND RESULTS PO Box 743427 Dallas, TX 75374 (888) 812-3577 Fax (972) 934-3091 IMPORTANT NOTICE FOR REQUIRED FILING FORMS DWC FORM-5 & DWC FORM-7 Caprock
IRS DATA RETRIEVAL NOTIFICATION DEPENDENT STUDENT ESTIMATOR
 IRS DATA RETRIEVAL NOTIFICATION DEPENDENT STUDENT ESTIMATOR Subject: Important Updates Needed for Your FAFSA Dear [Applicant], When you completed your 2012-2013 Free Application for Federal Student Aid
IRS DATA RETRIEVAL NOTIFICATION DEPENDENT STUDENT ESTIMATOR Subject: Important Updates Needed for Your FAFSA Dear [Applicant], When you completed your 2012-2013 Free Application for Federal Student Aid
Some examples. I wash my clothes, I wash the dishes, I wash the car, I wash the windows. I wash my hands, I wash my hair, I wash my face.
 Reflexive verbs In this presentation, we are going to look at a special group of verbs called reflexives. Let s start out by thinking of the English verb wash. List several things that you can wash. Some
Reflexive verbs In this presentation, we are going to look at a special group of verbs called reflexives. Let s start out by thinking of the English verb wash. List several things that you can wash. Some
Lump Sum Final Check Contribution to Deferred Compensation
 Memo To: ERF Members The Employees Retirement Fund has been asked by Deferred Compensation to provide everyone that has signed up to retire with the attached information. Please read the information from
Memo To: ERF Members The Employees Retirement Fund has been asked by Deferred Compensation to provide everyone that has signed up to retire with the attached information. Please read the information from
Condiciones y Reglas de la presentación de Declaración Sumaria de Salida (EXS)
 C/ Santa María Magdalena 16, 28016 Madrid ECS Sistema de Control de Exportaciones Condiciones y Reglas de la presentación de Declaración Sumaria de Salida (EXS) Autor: S.G.A.A Fecha: 06/06/2011 Versión:
C/ Santa María Magdalena 16, 28016 Madrid ECS Sistema de Control de Exportaciones Condiciones y Reglas de la presentación de Declaración Sumaria de Salida (EXS) Autor: S.G.A.A Fecha: 06/06/2011 Versión:
FCC Information : Warning: RF warning statement:
 FCC Information : This device complies with Part 15 of the FCC Rules. Operation is subject to the following two conditions: (1) This device may not cause harmful interference, and (2) This device must
FCC Information : This device complies with Part 15 of the FCC Rules. Operation is subject to the following two conditions: (1) This device may not cause harmful interference, and (2) This device must
UPV software for risk models
 UPV software for risk models Armando Serrano Lombillo arserlom@doctor.upv.es www.edams.upv.es Risk analysis is feasible 1 Contents Introduction Review of previous concepts From influence diagrams to event
UPV software for risk models Armando Serrano Lombillo arserlom@doctor.upv.es www.edams.upv.es Risk analysis is feasible 1 Contents Introduction Review of previous concepts From influence diagrams to event
Hígado bioartificial Revisión sistemática
 CONSEJERÍA DE IGUALDAD, SALUD Y POLÍTICAS SOCIALES AGENCIA DE EVALUACIÓN DE TECNOLOGÍAS SANITARIAS DE ANDALUCÍA (AETSA) Hígado bioartificial Revisión sistemática Informe de evaluación de tecnologías emergentes
CONSEJERÍA DE IGUALDAD, SALUD Y POLÍTICAS SOCIALES AGENCIA DE EVALUACIÓN DE TECNOLOGÍAS SANITARIAS DE ANDALUCÍA (AETSA) Hígado bioartificial Revisión sistemática Informe de evaluación de tecnologías emergentes
manual de servicio nissan murano z51
 manual de servicio nissan murano z51 Reference Manual To understand featuring to use and how to totally exploit manual de servicio nissan murano z51 to your great advantage, there are several sources of
manual de servicio nissan murano z51 Reference Manual To understand featuring to use and how to totally exploit manual de servicio nissan murano z51 to your great advantage, there are several sources of
ESTUDIO CLÍNICO CON IMPLANTES DE SUPERFICIE OXALIFE EN PACIENTES FUMADORES Y NO FUMADORES
 ESTUDIO CLÍNICO CON IMPLANTES DE SUPERFICIE OXALIFE EN PACIENTES FUMADORES Y NO FUMADORES OXALIFE IMPLANTS SURFACE IN SMOKING AND NO SMOKING PATIENTS: A CLINICAL STUDY Autor: Malbos Marisel Director: Ibáñez
ESTUDIO CLÍNICO CON IMPLANTES DE SUPERFICIE OXALIFE EN PACIENTES FUMADORES Y NO FUMADORES OXALIFE IMPLANTS SURFACE IN SMOKING AND NO SMOKING PATIENTS: A CLINICAL STUDY Autor: Malbos Marisel Director: Ibáñez
Adobe Acrobat Reader X: Manual to Verify the Digital Certification of a Document
 dobe crobat Reader X: Manual de verificación de Certificación Digital de un documento dobe crobat Reader X: Manual to Verify the Digital Certification of a Document support@bioesign.com Desarrollado por:
dobe crobat Reader X: Manual de verificación de Certificación Digital de un documento dobe crobat Reader X: Manual to Verify the Digital Certification of a Document support@bioesign.com Desarrollado por:
Level 1 Spanish, 2013
 90911 909110 1SUPERVISOR S Level 1 Spanish, 2013 90911 Demonstrate understanding of a variety of Spanish texts on areas of most immediate relevance 9.30 am Tuesday 3 December 2013 Credits: Five Achievement
90911 909110 1SUPERVISOR S Level 1 Spanish, 2013 90911 Demonstrate understanding of a variety of Spanish texts on areas of most immediate relevance 9.30 am Tuesday 3 December 2013 Credits: Five Achievement
Edgar Quiñones. HHRR: Common Sense Does Not Mean Business. Objective
 Edgar Quiñones HHRR: Common Sense Does Not Mean Business Objective Share experiences & insight gained in the last two decades in the management consulting business regarding why Common Sense Does Not Mean
Edgar Quiñones HHRR: Common Sense Does Not Mean Business Objective Share experiences & insight gained in the last two decades in the management consulting business regarding why Common Sense Does Not Mean
An explanation by Sr. Jordan
 & An explanation by Sr. Jdan direct object pronouns We usually use Direct Object Pronouns to substitute f it them in a sentence when the it them follows the verb. Because of gender, him and her could also
& An explanation by Sr. Jdan direct object pronouns We usually use Direct Object Pronouns to substitute f it them in a sentence when the it them follows the verb. Because of gender, him and her could also
Los números. 0 cero 1 uno / un 2 dos 3 tres 4 cuatro. 6 seis 7 siete 8 ocho 9 nueve 10 diez 5 cinco
 53 31 16 0 cero 1 uno / un 2 dos 3 tres 4 cuatro 6 seis 7 siete 8 ocho 9 nueve 10 diez 5 cinco 11 - once 12 - doce 13 - trece 14 - catorce 17 - diecisiete 18 - dieciocho 19 - diecinueve 20 - veinte 15
53 31 16 0 cero 1 uno / un 2 dos 3 tres 4 cuatro 6 seis 7 siete 8 ocho 9 nueve 10 diez 5 cinco 11 - once 12 - doce 13 - trece 14 - catorce 17 - diecisiete 18 - dieciocho 19 - diecinueve 20 - veinte 15
Self-Test Log Book. FOR RECORDING OF: Blood Glucose Test Results Insulin and Medication Doses Notes
 Self-Test Log Book FOR RECORDING OF: Blood Glucose Test Results Insulin and Medication Doses Notes When you see the Prestige Smart System brand identification on blood glucose monitors, test strips, blood
Self-Test Log Book FOR RECORDING OF: Blood Glucose Test Results Insulin and Medication Doses Notes When you see the Prestige Smart System brand identification on blood glucose monitors, test strips, blood
School Preference through the Infinite Campus Parent Portal
 School Preference through the Infinite Campus Parent Portal Welcome New and Returning Families! Enrollment for new families or families returning to RUSD after being gone longer than one year is easy.
School Preference through the Infinite Campus Parent Portal Welcome New and Returning Families! Enrollment for new families or families returning to RUSD after being gone longer than one year is easy.
IntesisBox PA-RC2-xxx-1 Panasonic compatibilities
 IntesisBox PA-RC2-xxx-1 Panasonic compatibilities In this document the compatible Panasonic models with the following IntesisBox RC2 interfaces are listed: / En éste documento se listan los modelos PANASONIC
IntesisBox PA-RC2-xxx-1 Panasonic compatibilities In this document the compatible Panasonic models with the following IntesisBox RC2 interfaces are listed: / En éste documento se listan los modelos PANASONIC
Tesis de Maestría titulada
 Tesis de Maestría titulada EL ANALISIS DE CONFIABILIDAD COMO HERRAMIENTA PARA OPTIMIZAR LA GESTIÓN DEL MANTENIMIENTO DE LOS EQUIPOS DE LA LÍNEA DE FLOTACIÓN EN UN CENTRO MINERO RESUMEN En la presente investigación
Tesis de Maestría titulada EL ANALISIS DE CONFIABILIDAD COMO HERRAMIENTA PARA OPTIMIZAR LA GESTIÓN DEL MANTENIMIENTO DE LOS EQUIPOS DE LA LÍNEA DE FLOTACIÓN EN UN CENTRO MINERO RESUMEN En la presente investigación
Terapia de campo de tumores en el tratamiento del glioblastoma
 Terapia de campo de tumores en el tratamiento del glioblastoma Revisión sistemática Informe de síntesis de tecnología emergente Tumor treating fields therapy (TTF) for glioblastoma. A Systematic Review
Terapia de campo de tumores en el tratamiento del glioblastoma Revisión sistemática Informe de síntesis de tecnología emergente Tumor treating fields therapy (TTF) for glioblastoma. A Systematic Review
AUTHORIZATION FOR USE OR DISCLOSURE OF HEALTH INFORMATION
 FORM 16-1 AUTHORIZATION FOR USE OR DISCLOSURE OF HEALTH INFORMATION Completion of this document authorizes the disclosure and use of health information about you. Failure to provide all information requested
FORM 16-1 AUTHORIZATION FOR USE OR DISCLOSURE OF HEALTH INFORMATION Completion of this document authorizes the disclosure and use of health information about you. Failure to provide all information requested
Bone Marrow Biopsy. To Prepare
 Bone Marrow Biopsy Bone marrow is the soft tissue inside your bones where blood cells are made. During a bone marrow biopsy, a small tissue sample is taken and sent for tests. The biopsy is often taken
Bone Marrow Biopsy Bone marrow is the soft tissue inside your bones where blood cells are made. During a bone marrow biopsy, a small tissue sample is taken and sent for tests. The biopsy is often taken
Nueva confirmación de pedido de compra con cambios: proveedor ES
 Ayuda de trabajo Nueva confirmación de pedido de compra con cambios: proveedor ES Step 1. This Supplier portal activity lists the steps necessary for confirming a new purchase order with changes on price,
Ayuda de trabajo Nueva confirmación de pedido de compra con cambios: proveedor ES Step 1. This Supplier portal activity lists the steps necessary for confirming a new purchase order with changes on price,
Telling Time in Spanish Supplemental Hand-out
 DSC ACADEMIC SUPPORT CENTER SPANISH WORKSHOPS STUDENT HANDOUT Telling Time in Spanish Supplemental Hand-out To ask someone the time in Spanish, you say: Qué hora es? - What time is it? To tell the time
DSC ACADEMIC SUPPORT CENTER SPANISH WORKSHOPS STUDENT HANDOUT Telling Time in Spanish Supplemental Hand-out To ask someone the time in Spanish, you say: Qué hora es? - What time is it? To tell the time
Sierra Security System
 Using Your SpreadNet Accessories With Your Sierra Security System Uso de Sus Accesorios SpreadNet Con Su Sistema de Seguridad Sierra SN990-KEYPAD SN961-KEYFOB SN991-REMOTE 1 SN990-KEYPAD The SN990-KEYPAD
Using Your SpreadNet Accessories With Your Sierra Security System Uso de Sus Accesorios SpreadNet Con Su Sistema de Seguridad Sierra SN990-KEYPAD SN961-KEYFOB SN991-REMOTE 1 SN990-KEYPAD The SN990-KEYPAD
Tema: Study for the Analysis and the Conceptual Development of a European Port Access System. Ponente: Mario J. Moya Denia
 Tema: Study for the Analysis and the Conceptual Development of a European Port Access System Introduction EPAIC I (European Port Access Identification Card) study, completed in 2008, concluded that there
Tema: Study for the Analysis and the Conceptual Development of a European Port Access System Introduction EPAIC I (European Port Access Identification Card) study, completed in 2008, concluded that there
Conditioning Exercises: Standing
 Conditioning Exercises: Standing Do all these exercises slowly. Do not hold your breath during these exercises. If unusual pain occurs in your joints or muscles while you are exercising, do not continue
Conditioning Exercises: Standing Do all these exercises slowly. Do not hold your breath during these exercises. If unusual pain occurs in your joints or muscles while you are exercising, do not continue
Pregunta 1 Suponga que una muestra de 35 observaciones es obtenida de una población con media y varianza. Entonces la se calcula como.
 Universidad de Costa Rica Programa de Posgrado en Computación e Informática Doctorado en Computación e Informática Curso Estadística 18 de febrero 2013 Nombre: Segundo examen corto de Probabilidad Pregunta
Universidad de Costa Rica Programa de Posgrado en Computación e Informática Doctorado en Computación e Informática Curso Estadística 18 de febrero 2013 Nombre: Segundo examen corto de Probabilidad Pregunta
Does Traction Help a Bent Member?
 Does Traction Help a Bent Member? The appearance of a guy s member can be a matter of great concern to a man. Every guy wants to sport a handsome male organ, and so men may spend time admiring or worrying
Does Traction Help a Bent Member? The appearance of a guy s member can be a matter of great concern to a man. Every guy wants to sport a handsome male organ, and so men may spend time admiring or worrying
Instructor: Do you remember how to say the verb "to speak"? Instructor: How do you ask a friend Do you speak Spanish?
 Learning Spanish Like Crazy Spoken Spanish Lección Dos. Listen to the following conversation: Male: Hablas inglés? Female: Sí, hablo inglés porque practico todos los días. Male: Dónde? Female: Practico
Learning Spanish Like Crazy Spoken Spanish Lección Dos. Listen to the following conversation: Male: Hablas inglés? Female: Sí, hablo inglés porque practico todos los días. Male: Dónde? Female: Practico
DIAMOND Gear Company, LTD. an ERIKS Company. Installation, Maintenance, & Operation Manual DECLUTCHABLE WORM GEAR
 DIAMOND Gear Company, LTD. an ERIKS Company Installation, Maintenance, & Operation Manual 2013 INSTRUCTIONS This is an instructional manual which provides general installation, operation, and maintenance
DIAMOND Gear Company, LTD. an ERIKS Company Installation, Maintenance, & Operation Manual 2013 INSTRUCTIONS This is an instructional manual which provides general installation, operation, and maintenance
Examen prenatal: Evaluación integrada En la Clínica de Medicina Materno Fetal en Yakima
 UW MEDICINE PATIENT EDUCATION PRENATAL TESTING: INTEGRATED SCREEN SPANISH Examen prenatal: Evaluación integrada En la Clínica de Medicina Materno Fetal en Yakima En la Clínica de Medicina Materno Fetal
UW MEDICINE PATIENT EDUCATION PRENATAL TESTING: INTEGRATED SCREEN SPANISH Examen prenatal: Evaluación integrada En la Clínica de Medicina Materno Fetal en Yakima En la Clínica de Medicina Materno Fetal
Agustiniano Ciudad Salitre School Computer Science Support Guide - 2015 Second grade First term
 Agustiniano Ciudad Salitre School Computer Science Support Guide - 2015 Second grade First term UNIDAD TEMATICA: INTERFAZ DE WINDOWS LOGRO: Reconoce la interfaz de Windows para ubicar y acceder a los programas,
Agustiniano Ciudad Salitre School Computer Science Support Guide - 2015 Second grade First term UNIDAD TEMATICA: INTERFAZ DE WINDOWS LOGRO: Reconoce la interfaz de Windows para ubicar y acceder a los programas,
Efectividad y seguridad de la artroplastia total discal en lumbalgia crónica. Revisión sistemática y metaanálisis
 Efectividad y seguridad de la artroplastia total discal en lumbalgia crónica. Revisión sistemática y metaanálisis Lumbar total disc replacement effectiveness and safety in chronic low back pain. Executive
Efectividad y seguridad de la artroplastia total discal en lumbalgia crónica. Revisión sistemática y metaanálisis Lumbar total disc replacement effectiveness and safety in chronic low back pain. Executive
http://mvision.madrid.org
 Apoyando el desarrollo de carrera de investigadores en imagen biomédica Supporting career development of researchers in biomedical imaging QUÉ ES M+VISION? WHAT IS M+VISION? M+VISION es un programa creado
Apoyando el desarrollo de carrera de investigadores en imagen biomédica Supporting career development of researchers in biomedical imaging QUÉ ES M+VISION? WHAT IS M+VISION? M+VISION es un programa creado
Steps to Understand Your Child s Behavior. Customizing the Flyer
 Steps to Understand Your Child s Behavior Customizing the Flyer Hello! Here is the PDF Form Template for use in advertising Steps to Understanding Your Child s Behavior (HDS Behavior Level 1B). Because
Steps to Understand Your Child s Behavior Customizing the Flyer Hello! Here is the PDF Form Template for use in advertising Steps to Understanding Your Child s Behavior (HDS Behavior Level 1B). Because
Health in Peru, 1991-2003. Prepared by Leigh Campoamor
 Prepared by Leigh Campoamor Princeton University Library Princeton, NJ 2003 Scope Note Contents: This collection contains pamphlets, articles, and other miscellaneous items addressing a range of health-related
Prepared by Leigh Campoamor Princeton University Library Princeton, NJ 2003 Scope Note Contents: This collection contains pamphlets, articles, and other miscellaneous items addressing a range of health-related
Instructor: She just said that she s Puerto Rican. Escucha y repite la palabra Puerto Rican -for a man-.
 Learning Spanish Like Crazy Spoken Spanish Lección once Instructor: Cómo se dice Good afternoon? René: Buenas tardes. Buenas tardes. Instructor: How do you ask a woman if she s Colombian. René: Eres Colombiana?
Learning Spanish Like Crazy Spoken Spanish Lección once Instructor: Cómo se dice Good afternoon? René: Buenas tardes. Buenas tardes. Instructor: How do you ask a woman if she s Colombian. René: Eres Colombiana?
LAC-2009-09 Modificación 2.3.3.3. DIRECT ALLOCATIONS TO ISPs DISTRIBUCIONES INICIALES A ISPs
 LAC-2009-09 Modificación 2.3.3.3 DIRECT ALLOCATIONS TO ISPs DISTRIBUCIONES INICIALES A ISPs Current Policy 2.3.3.3. Direct Allocations to Internet Service Providers LACNIC may grant this type of allocation
LAC-2009-09 Modificación 2.3.3.3 DIRECT ALLOCATIONS TO ISPs DISTRIBUCIONES INICIALES A ISPs Current Policy 2.3.3.3. Direct Allocations to Internet Service Providers LACNIC may grant this type of allocation
The Home Language Survey (HLS) and Identification of Students
 The Home Language Survey (HLS) and Identification of Students The Home Language Survey (HLS) is the document used to determine a student that speaks a language other than English. Identification of a language
The Home Language Survey (HLS) and Identification of Students The Home Language Survey (HLS) is the document used to determine a student that speaks a language other than English. Identification of a language
ADAPTACIÓN DE REAL TIME WORKSHOP AL SISTEMA OPERATIVO LINUX
 ADAPTACIÓN DE REAL TIME WORKSHOP AL SISTEMA OPERATIVO LINUX Autor: Tomás Murillo, Fernando. Director: Muñoz Frías, José Daniel. Coordinador: Contreras Bárcena, David Entidad Colaboradora: ICAI Universidad
ADAPTACIÓN DE REAL TIME WORKSHOP AL SISTEMA OPERATIVO LINUX Autor: Tomás Murillo, Fernando. Director: Muñoz Frías, José Daniel. Coordinador: Contreras Bárcena, David Entidad Colaboradora: ICAI Universidad
Level 1 Spanish, 2014
 90911 909110 1SUPERVISOR S Level 1 Spanish, 2014 90911 Demonstrate understanding of a variety of Spanish texts on areas of most immediate relevance 2.00 pm Friday 28 November 2014 Credits: Five Achievement
90911 909110 1SUPERVISOR S Level 1 Spanish, 2014 90911 Demonstrate understanding of a variety of Spanish texts on areas of most immediate relevance 2.00 pm Friday 28 November 2014 Credits: Five Achievement
Lengua adicional al español IV
 Lengua adicional al español IV Topic 11 Life little lessons Introduction In this lesson you will study: Time clauses are independent clauses. These are the clauses that tell you the specific time when
Lengua adicional al español IV Topic 11 Life little lessons Introduction In this lesson you will study: Time clauses are independent clauses. These are the clauses that tell you the specific time when
Chattanooga Motors - Solicitud de Credito
 Chattanooga Motors - Solicitud de Credito Completa o llena la solicitud y regresala en persona o por fax. sotros mantenemos tus datos en confidencialidad. Completar una aplicacion para el comprador y otra
Chattanooga Motors - Solicitud de Credito Completa o llena la solicitud y regresala en persona o por fax. sotros mantenemos tus datos en confidencialidad. Completar una aplicacion para el comprador y otra
EMPLOYER & EMPLOYEE RETIREMENT PLAN TAX CREDITS
 EMPLOYER & EMPLOYEE RETIREMENT PLAN TAX CREDITS For employers who set up and maintain retirement plans, the setup costs, annual administrative costs, and retirement-related employee education costs are
EMPLOYER & EMPLOYEE RETIREMENT PLAN TAX CREDITS For employers who set up and maintain retirement plans, the setup costs, annual administrative costs, and retirement-related employee education costs are
News Flash! Primary & Specialty Care Providers. Sharp Health Plan. Date: February 17, 2012. Subject: Member Grievance Forms
 I M P O R T A N T News Flash! A FAX Publication for Providers of Sharp Health Plan To: From: Primary & Specialty Care Providers Sharp Health Plan Date: February 17, 2012 Subject: Member Grievance Forms
I M P O R T A N T News Flash! A FAX Publication for Providers of Sharp Health Plan To: From: Primary & Specialty Care Providers Sharp Health Plan Date: February 17, 2012 Subject: Member Grievance Forms
PEMEX E&P South Region OMC 2015
 PEMEX E&P South Region OMC 2015 Austin, TX. USA Por: Mario Alejandro Mosqueda Thompson (PEMEX) Juan D. Osorio Monsalve (OVS GROUP MEXICO) The system is based on integrating databases and capture of different
PEMEX E&P South Region OMC 2015 Austin, TX. USA Por: Mario Alejandro Mosqueda Thompson (PEMEX) Juan D. Osorio Monsalve (OVS GROUP MEXICO) The system is based on integrating databases and capture of different
Reinforcement Plan. Day 27 Month 03 Year 2015
 BETHLEMITAS SCHOOL Reinforcement Plan Day 27 Month 03 Year 2015 TERM: I Date: COMPREHENSION GOAL: The students develop comprehension about the Living and Non- living things, plants, animals and their main
BETHLEMITAS SCHOOL Reinforcement Plan Day 27 Month 03 Year 2015 TERM: I Date: COMPREHENSION GOAL: The students develop comprehension about the Living and Non- living things, plants, animals and their main
ANÁLISIS DE IMÁGENES: SISTEMA DE SEGUIMIENTO DE PERSONAS. Arranz Domingo, Álvaro. Sánchez Miralles, Álvaro.
 ANÁLISIS DE IMÁGENES: SISTEMA DE SEGUIMIENTO DE PERSONAS Autor: Directores: Garzón González, Elena. Alvar Miró, Manuel. Arranz Domingo, Álvaro. Sánchez Miralles, Álvaro. Entidad Colaboradora: ICAI - Universidad
ANÁLISIS DE IMÁGENES: SISTEMA DE SEGUIMIENTO DE PERSONAS Autor: Directores: Garzón González, Elena. Alvar Miró, Manuel. Arranz Domingo, Álvaro. Sánchez Miralles, Álvaro. Entidad Colaboradora: ICAI - Universidad
CON BOLTEX INERTIAL HOW TO EXERCISE WITH BOLTEX INERTIAL COMMENT UTILISER BOLTEX INERTIAL
 3CÓMO EJERCITAR CON BOLTEX INERTIAL HOW TO EXERCISE WITH BOLTEX INERTIAL COMMENT UTILISER BOLTEX INERTIAL Indicaciones de Uso Instructions for Use / Instructions d Utilisation Tratamiento de la incontinencia
3CÓMO EJERCITAR CON BOLTEX INERTIAL HOW TO EXERCISE WITH BOLTEX INERTIAL COMMENT UTILISER BOLTEX INERTIAL Indicaciones de Uso Instructions for Use / Instructions d Utilisation Tratamiento de la incontinencia
CONSENT FOR HIV BLOOD TEST
 i have been informed that a sample of my blood will be obtained and tested to determine the presence of antibodies to human immunodeficiency Virus (hiv), the virus that causes Acquired immune Deficiency
i have been informed that a sample of my blood will be obtained and tested to determine the presence of antibodies to human immunodeficiency Virus (hiv), the virus that causes Acquired immune Deficiency
UNIVERSIDAD DE GUAYAQUIL FACULTAD DE ODONTOLOGÍA ESCUELA DE POSTGRADO Dr. José Apolo Pineda
 UNIVERSIDAD DE GUAYAQUIL FACULTAD DE ODONTOLOGÍA ESCUELA DE POSTGRADO Dr. José Apolo Pineda EVALUACIÓN IN VITRO DE LA FILTRACIÓN APICAL EN RAICES DE DIENTES EXTRAIDOS, UTILIZANDO DOS MÉTODOS DE OBTURACION:
UNIVERSIDAD DE GUAYAQUIL FACULTAD DE ODONTOLOGÍA ESCUELA DE POSTGRADO Dr. José Apolo Pineda EVALUACIÓN IN VITRO DE LA FILTRACIÓN APICAL EN RAICES DE DIENTES EXTRAIDOS, UTILIZANDO DOS MÉTODOS DE OBTURACION:
Dolores de cabeza Trabaje con su doctor para evitar las visitas a la Sala de Emergencia
 Headaches, Working with your Doctor to Avoid the Emergency Room Dolores de cabeza Trabaje con su doctor para evitar las visitas a la Sala de Emergencia Conozca a su equipo de cuidados para los dolores
Headaches, Working with your Doctor to Avoid the Emergency Room Dolores de cabeza Trabaje con su doctor para evitar las visitas a la Sala de Emergencia Conozca a su equipo de cuidados para los dolores
"Average years of healthy life (without disabilities). Argentina 2002-2003.
 Universidad Nacional de Córdoba "Average years of healthy life (without disabilities). Argentina 2002-2003. Alvarez, M. - Carrizo, E. - Peláez, E. - González, L. CONICET - National University of Córdoba,
Universidad Nacional de Córdoba "Average years of healthy life (without disabilities). Argentina 2002-2003. Alvarez, M. - Carrizo, E. - Peláez, E. - González, L. CONICET - National University of Córdoba,
Learning Masters. Fluent: States of Matter
 Learning Masters Fluent: States of Matter What I Learned List the three most important things you learned in this theme. Tell why you listed each one. 1. 2. 3. 22 States of Matter Learning Masters How
Learning Masters Fluent: States of Matter What I Learned List the three most important things you learned in this theme. Tell why you listed each one. 1. 2. 3. 22 States of Matter Learning Masters How
Spanish 3V: Winter 2014
 Spanish 3V: Winter 2014 Elementary Spanish 3 in online format: https://login.uconline.edu/ Robert Blake, rjblake@ucdavis.edu; Rebecca Conley, mconley@ucdavis.edu Description: Spanish 3V is the second of
Spanish 3V: Winter 2014 Elementary Spanish 3 in online format: https://login.uconline.edu/ Robert Blake, rjblake@ucdavis.edu; Rebecca Conley, mconley@ucdavis.edu Description: Spanish 3V is the second of
GENERAL INFORMATION Project Description
 RESULTADOS! GENERAL INFORMATION Project Description The campaign "Adopt a car " had as its main objective to position Autoplaza, the main automotive selling point of Chile, as a new car sales location
RESULTADOS! GENERAL INFORMATION Project Description The campaign "Adopt a car " had as its main objective to position Autoplaza, the main automotive selling point of Chile, as a new car sales location
ID. Number: 40-01 Su Seguridad y Salud al Soldar o Cortar / Your Safety and Health When Welding or Cutting
 CATEGORIA 40 SOLDADURA/WELDING ID. Number: 40-01 Su Seguridad y Salud al Soldar o Cortar / Your Safety and Health When Welding or Cutting Produced: Publication date: Literacy level: Appropriate for: OHSP
CATEGORIA 40 SOLDADURA/WELDING ID. Number: 40-01 Su Seguridad y Salud al Soldar o Cortar / Your Safety and Health When Welding or Cutting Produced: Publication date: Literacy level: Appropriate for: OHSP
Questionnaires for the Evaluation of Awareness in a Groupware Application
 Questionnaires for the Evaluation of Awareness in a Groupware Application Technical Report DIAB-12-11-1 Montserrat Sendín a, Juan-Miguel López-Gil b, and Víctor López-Jaquero c a GRIHO HCI Research Lab.,
Questionnaires for the Evaluation of Awareness in a Groupware Application Technical Report DIAB-12-11-1 Montserrat Sendín a, Juan-Miguel López-Gil b, and Víctor López-Jaquero c a GRIHO HCI Research Lab.,
Sistema basado en firma digital para enviar datos por Internet de forma segura mediante un navegador.
 Sistema basado en firma digital para enviar datos por Internet de forma segura mediante un navegador. Autor: David de la Fuente González Directores: Rafael Palacios, Javier Jarauta. Este proyecto consiste
Sistema basado en firma digital para enviar datos por Internet de forma segura mediante un navegador. Autor: David de la Fuente González Directores: Rafael Palacios, Javier Jarauta. Este proyecto consiste
Severe Male Organ Pain: Is it a Nerve Problem?
 Severe Male Organ Pain: Is it a Nerve Problem? All those sensitive nerves in the member are the reason that it feels so good when the member is stroked, enveloped or otherwise made the subject of physical
Severe Male Organ Pain: Is it a Nerve Problem? All those sensitive nerves in the member are the reason that it feels so good when the member is stroked, enveloped or otherwise made the subject of physical
Creating your Single Sign-On Account for the PowerSchool Parent Portal
 Creating your Single Sign-On Account for the PowerSchool Parent Portal Welcome to the Parent Single Sign-On. What does that mean? Parent Single Sign-On offers a number of benefits, including access to
Creating your Single Sign-On Account for the PowerSchool Parent Portal Welcome to the Parent Single Sign-On. What does that mean? Parent Single Sign-On offers a number of benefits, including access to
UTILIZACIÓN DE UN BOLÍGRAFO DÍGITAL PARA LA MEJORA DE PROCEDIMIENTOS DE CAMPO EN UNA CENTRAL NUCLEAR.
 UTILIZACIÓN DE UN BOLÍGRAFO DÍGITAL PARA LA MEJORA DE PROCEDIMIENTOS DE CAMPO EN UNA CENTRAL NUCLEAR. Autor: Ruiz Muñoz, Rafael. Director: Muñoz García, Manuel. Entidad Colaboradora: Empresarios Agrupados.
UTILIZACIÓN DE UN BOLÍGRAFO DÍGITAL PARA LA MEJORA DE PROCEDIMIENTOS DE CAMPO EN UNA CENTRAL NUCLEAR. Autor: Ruiz Muñoz, Rafael. Director: Muñoz García, Manuel. Entidad Colaboradora: Empresarios Agrupados.
Affordable Care Act Informative Sessions and Open Enrollment Event
 2600 Cedar Ave., P.O. Box 2337, Laredo, TX 78044 Hector F. Gonzalez, M.D., M.P.H Tel. (956) 795-4901 Fax. (956) 726-2632 Director of Health News Release. Date: February 9, 2015 FOR IMMEDIATE RELEASE To:
2600 Cedar Ave., P.O. Box 2337, Laredo, TX 78044 Hector F. Gonzalez, M.D., M.P.H Tel. (956) 795-4901 Fax. (956) 726-2632 Director of Health News Release. Date: February 9, 2015 FOR IMMEDIATE RELEASE To:
UNIT 9.- INTRODUCTION TO HYPOTHESIS TESTING.
 STATISTICAL METHODS FOR BUSINESS UNIT 9.- INTRODUCTION TO HYPOTHESIS TESTING. 9.1.- Basics of statistical hypothesis testing. 9.2.- Types of errors in hypothesis testing. 9.3.- Methodology and implementation
STATISTICAL METHODS FOR BUSINESS UNIT 9.- INTRODUCTION TO HYPOTHESIS TESTING. 9.1.- Basics of statistical hypothesis testing. 9.2.- Types of errors in hypothesis testing. 9.3.- Methodology and implementation
GOLDCORP INC. GOLD AND SILVER RESERVES AND RESOURCES SUMMARY TABLE As of December 31, 2014
 . GOLD AND SILVER RESERVES AND RESOURCES SUMMARY TABLE GOLDCORP INC PROVEN AND PROBABLE RESERVES (1)(4)(5) Alumbrera (37.5%) Argentina 56.25 0.31 0.55 Camino Rojo Mexico 84.52 0.68 1.85 Cerro Negro Argentina
. GOLD AND SILVER RESERVES AND RESOURCES SUMMARY TABLE GOLDCORP INC PROVEN AND PROBABLE RESERVES (1)(4)(5) Alumbrera (37.5%) Argentina 56.25 0.31 0.55 Camino Rojo Mexico 84.52 0.68 1.85 Cerro Negro Argentina
Specimen 2018 Morning Time allowed: 1 hour 15 minutes
 SPECIMEN MATERIAL GCSE SPANISH Higher Tier Paper 4 Writing H Specimen 2018 Morning Time allowed: 1 hour 15 minutes Materials: You will need no other materials. Instructions Use black ink or black ball-point
SPECIMEN MATERIAL GCSE SPANISH Higher Tier Paper 4 Writing H Specimen 2018 Morning Time allowed: 1 hour 15 minutes Materials: You will need no other materials. Instructions Use black ink or black ball-point
From e-pedagogies to activity planners. How can it help a teacher?
 From e-pedagogies to activity planners. How can it help a teacher? Elena de Miguel, Covadonga López, Ana Fernández-Pampillón & Maria Matesanz Universidad Complutense de Madrid ABSTRACT Within the framework
From e-pedagogies to activity planners. How can it help a teacher? Elena de Miguel, Covadonga López, Ana Fernández-Pampillón & Maria Matesanz Universidad Complutense de Madrid ABSTRACT Within the framework
Male Organ Function and the Impact of Diabetes
 Male Organ Function and the Impact of Diabetes More than 30 million Americas 9.4% of the population are thought to have diabetes and about a quarter of them don t know it. Equally alarming, some 84 million
Male Organ Function and the Impact of Diabetes More than 30 million Americas 9.4% of the population are thought to have diabetes and about a quarter of them don t know it. Equally alarming, some 84 million
Bent Male Organ After Using a Pump: How and Why it Happens
 Bent Male Organ After Using a Pump: How and Why it Happens The male organ pump is a device which many men with tumescence issues use to help overcome these issues, and as such can be a valuable male organ
Bent Male Organ After Using a Pump: How and Why it Happens The male organ pump is a device which many men with tumescence issues use to help overcome these issues, and as such can be a valuable male organ
SIGUIENDO LOS REQUISITOS ESTABLECIDOS EN LA NORMA ISO 14001 Y CONOCIENDO LAS CARACTERISTICAS DE LA EMPRESA CARTONAJES MIGUEL Y MATEO EL ALUMNO DEBERA
 SIGUIENDO LOS REQUISITOS ESTABLECIDOS EN LA NORMA ISO 14001 Y CONOCIENDO LAS CARACTERISTICAS DE LA EMPRESA CARTONAJES MIGUEL Y MATEO EL ALUMNO DEBERA ELABORAR LA POLITICA AMBIENTAL PDF File: Siguiendo
SIGUIENDO LOS REQUISITOS ESTABLECIDOS EN LA NORMA ISO 14001 Y CONOCIENDO LAS CARACTERISTICAS DE LA EMPRESA CARTONAJES MIGUEL Y MATEO EL ALUMNO DEBERA ELABORAR LA POLITICA AMBIENTAL PDF File: Siguiendo
Flashcards Series 3 El Aeropuerto
 Flashcards Series 3 El Aeropuerto Flashcards are one of the quickest and easiest ways to test yourself on Spanish vocabulary, no matter where you are! Test yourself on just these flashcards at first. Then,
Flashcards Series 3 El Aeropuerto Flashcards are one of the quickest and easiest ways to test yourself on Spanish vocabulary, no matter where you are! Test yourself on just these flashcards at first. Then,
Objetivo: Investigar los cambios ocurridos en la epidemiología clínica y molecular de A. baumannii entre 2000 y Método: Dos estudios
 1 2 3 and the GEIH/GEMARA/REIPI-Ab2010 Group. 4 Objetivo: Investigar los cambios ocurridos en la epidemiología clínica y molecular de A. baumannii entre 2000 y 2010. Método: Dos estudios prospectivos de
1 2 3 and the GEIH/GEMARA/REIPI-Ab2010 Group. 4 Objetivo: Investigar los cambios ocurridos en la epidemiología clínica y molecular de A. baumannii entre 2000 y 2010. Método: Dos estudios prospectivos de
IntesisBox MD-AC-xxx-yy AC indoor unit compatibilities
 IntesisBox MD-AC-xxx-yy AC indoor unit compatibilities In this document the compatible Midea Air conditioner indoor units models with the following IntesisBox AC interfaces are described: / En éste documento
IntesisBox MD-AC-xxx-yy AC indoor unit compatibilities In this document the compatible Midea Air conditioner indoor units models with the following IntesisBox AC interfaces are described: / En éste documento
b. Cuál es la razón principal por la que escogió esta respuesta?
 Creemos que es importante conocer la opinión de los pacientes sobre los estudios de investigación. Esto es especialmente importante en el estudio ESETT (Ensayo del tratamiento del estado epiléptico establecido)
Creemos que es importante conocer la opinión de los pacientes sobre los estudios de investigación. Esto es especialmente importante en el estudio ESETT (Ensayo del tratamiento del estado epiléptico establecido)
