International Nonproprietary Names for Pharmaceutical Substances (INN)
|
|
|
- Antonia Domínguez Camacho
- hace 6 años
- Vistas:
Transcripción
1 WHO Drug Information, Vol. 30, No. 4, 2016 Proposed INN: List 116 International Nonproprietary Names for Pharmaceutical Substances (INN) Notice is hereby given that, in accordance with article 3 of the Procedure for the Selection of Recommended International Nonproprietary Names for Pharmaceutical Substances, the names given in the list on the following pages are under consideration by the World Health Organization as Proposed International Nonproprietary Names. The inclusion of a name in the lists of Proposed International Nonproprietary Names does not imply any recommendation of the use of the substance in medicine or pharmacy. Lists of Proposed (1 113) and Recommended (1 74) International Nonproprietary Names can be found in Cumulative List No. 16, 2015 (available in CD-ROM only). The statements indicating action and use are based largely on information supplied by the manufacturer. This information is merely meant to provide an indication of the potential use of new substances at the time they are accorded Proposed International Nonproprietary Names. WHO is not in a position either to uphold these statements or to comment on the efficacy of the action claimed. Because of their provisional nature, these descriptors will neither be revised nor included in the Cumulative Lists of INNs. Dénominations communes internationales des Substances pharmaceutiques (DCI) Il est notifié que, conformément aux dispositions de l'article 3 de la Procédure à suivre en vue du choix de Dénominations communes internationales recommandées pour les Substances pharmaceutiques les dénominations ci-dessous sont mises à l'étude par l'organisation mondiale de la Santé en tant que dénominations communes internationales proposées. L'inclusion d'une dénomination dans les listes de DCI proposées n'implique aucune recommandation en vue de l'utilisation de la substance correspondante en médecine ou en pharmacie. On trouvera d'autres listes de Dénominations communes internationales proposées (1 113) et recommandées (1 74) dans la Liste récapitulative No. 16, 2015 (disponible sur CD-ROM seulement). Les mentions indiquant les propriétés et les indications des substances sont fondées sur les renseignements communiqués par le fabricant. Elles ne visent qu'à donner une idée de l'utilisation potentielle des nouvelles substances au moment où elles sont l'objet de propositions de DCI. L'OMS n'est pas en mesure de confirmer ces déclarations ni de faire de commentaires sur l'efficacité du mode d'action ainsi décrit. En raison de leur caractère provisoire, ces informations ne figureront pas dans les listes récapitulatives de DCI. Denominaciones Comunes Internacionales para las Sustancias Farmacéuticas (DCI) De conformidad con lo que dispone el párrafo 3 del "Procedimiento de Selección de Denominaciones Comunes Internacionales Recomendadas para las Sustancias Farmacéuticas", se comunica por el presente anuncio que las denominaciones detalladas en las páginas siguientes están sometidas a estudio por la Organización Mundial de La Salud como Denominaciones Comunes Internacionales Propuestas. La inclusión de una denominación en las listas de las DCI Propuestas no supone recomendación alguna en favor del empleo de la sustancia respectiva en medicina o en farmacia. Las listas de Denominaciones Comunes Internacionales Propuestas (1 113) y Recomendadas (1 74) se encuentran reunidas en Cumulative List No. 16, 2015 (disponible sólo en CD-ROM). Las indicaciones sobre acción y uso que aparecen se basan principalmente en la información facilitada por los fabricantes. Esta información tiene por objeto dar una idea únicamente de las posibilidades de aplicación de las nuevas sustancias a las que se asigna una DCI Propuesta. La OMS no está facultada para respaldar esas indicaciones ni para formular comentarios sobre la eficacia de la acción que se atribuye al producto. Debido a su carácter provisional, esos datos descriptivos no deben incluirse en las listas recapitulativas de DCI. 605
2 Proposed INN: List 116 WHO Drug Information, Vol. 30, No. 4, 2016 Proposed International Nonproprietary Names: List 116 Comments on, or formal objections to, the proposed names may be forwarded by any person to the INN Programme of the World Health Organization within four months of the date of their publication in WHO Drug Information, i.e., for List 116 Proposed INN not later than 02 May Publication date: 03/01/2017 Dénominations communes internationales proposées: Liste 116 Des observations ou des objections formelles à l'égard des dénominations proposées peuvent être adressées par toute personne au Programme des Dénominations communes internationales de l'organisation mondiale de la Santé dans un délai de quatre mois à compter de la date de leur publication dans WHO Drug Information, c'est à dire pour la Liste 116 de DCI Proposées le 02 mai 2017 au plus tard. Date de publication: 03/01/2017 Denominaciones Comunes Internacionales Propuestas: Lista 116 Cualquier persona puede dirigir observaciones u objeciones respecto de las denominaciones propuestas, al Programa de Denominaciones Comunes Internacionales de la Organización Mundial de la Salud, en un plazo de cuatro meses, contados desde la fecha de su publicación en WHO Drug Information, es decir, para la Lista 116 de DCI Propuestas el 02 de mayo de 2017 a más tardar. Fecha de publicación: 03/01/2017 Proposed INN (Latin, English, French, Spanish) DCI Proposée DCI Propuesta Chemical name or description: Action and use: Molecular formula Chemical Abstracts Service (CAS) registry number: Graphic formula Nom chimique ou description: Propriétés et indications: Formule brute Numéro dans le registre du CAS: Formule développée Nombre químico o descripción: Acción y uso: Fórmula molecular Número de registro del CAS: Fórmula desarrollada acoziborolum acoziborole acoziborole acoziborol 4-fluoro-N-(1-hydroxy-3,3-dimethyl-1,3-dihydro- 2,1-benzoxaborol-6-yl)-2-(trifluoromethyl)benzamide antiparasitic 4-fluoro-N-(1-hydroxy-3,3-diméthyl-1,3-dihydro- 2,1-benzoxaborol-6-yl)-2-(trifluorométhyl)benzamide antiparasitaire 4-fluoro-N-(1-hidroxi-3,3-dimetil-1,3-dihidro- 2,1-benzoxaborol-6-il)-2-(trifluorometil)benzamida antiparasitario C 17 H 14 BF 4 NO
3 WHO Drug Information, Vol. 30, No. 4, 2016 Proposed INN: List 116 acrizanibum acrizanib acrizanib acrizanib 5-({6-[(methylamino)methyl]pyrimidin-4-yl}oxy)- N-[1-methyl-5-(trifluoromethyl)-1H-pyrazol-3-yl]-1H-indole- 1-carboxamide angiogenesis inhibitor 5-({6-[(méthylamino)méthyl]pyrimidin-4-yl}oxy)- N-[1-méthyl-5-(trifluorométhyl)-1H-pyrazol-3-yl]-1H-indole- 1-carboxamide inhibiteur de l'angiogénèse 5-({6-[(metilamino)metil]pirimidin-4-il}oxi)-N-[1-metil- 5-(trifluorometil)-1H-pirazol-3-il]-1H-indol-1-carboxamida inhibidor de la angiogénesis C 20 H 18 F 3 N 7 O aprocitentanum aprocitentan aprocitentan aprocitentán N-[5-(4-bromophenyl)-6-{2-[(5-bromopyrimidin- 2-yl)oxy]ethoxy}pyrimidin-4-yl]sulfuric diamide endothelin receptor antagonist diamide N-[5-(4-bromophényl)-6-{2-[(5-bromopyrimidin- 2-yl)oxy]éthoxy}pyrimidin-4-yl]sulfurique antagoniste du récepteur de l'endothéline diamida N-[5-(4-bromofenil)-6-{2-[(5-bromopirimidin- 2-il)oxi]etoxi}pirimidin-4-il]sulfúrico antagonista del receptor de la endotelina C 16 H 14 Br 2 N 6 O 4 S atelocantelum atelocantel atélocantel (2E)-4,4-difluoro-N-{2-[(2-methoxypyridin- 4-yl)amino]ethyl}pent-2-enamide anthelmintic (veterinary drug) (2E)-4,4-difluoro-N-{2-[(2-méthoxypyridin- 4-yl)amino]éthyl}pent-2-enamide antihelmintique (usage vétérinaire) 607
4 Proposed INN: List 116 WHO Drug Information, Vol. 30, No. 4, 2016 atelocantel (2E)-4,4-difluoro-N-{2-[(2-metoxipiridin-4-il)amino]etil}pent- 2-enamida antihelmíntico (uso veterinario) C 13 H 17 F 2 N 3 O atesidorsenum atesidorsen atésidorsen all-p-ambo-2'-o-(2-methoxyethyl)-5-methyl-p-thiouridylyl- (3' 5')-2'-O-(2-methoxyethyl)-5-methyl-P-thiocytidylyl- (3' 5')-2'-O-(2-methoxyethyl)-P-thioadenylyl-(3' 5')-2'-O- (2-methoxyethyl)-P-thioguanylyl-(3' 5')-2'-O-(2- methoxyethyl)-p-thioguanylyl-(3' 5')-2'-deoxy-P- thioguanylyl-(3' 5')-2'-deoxy-5-methyl-P-thiocytidylyl- (3' 5')-2'-deoxy-P-thioadenylyl-(3' 5')-P-thiothymidylyl- (3' 5')-P-thiothymidylyl-(3' 5')-2'-deoxy-5-methyl-Pthiocytidylyl-(3' 5')-P-thiothymidylyl-(3' 5')-Pthiothymidylyl-(3' 5')-P-thiothymidylyl-(3' 5')-2'-deoxy-5- methyl-p-thiocytidylyl-(3' 5')-2'-O-(2-methoxyethyl)-5- methyl-p-thiocytidylyl-(3' 5')-2'-O-(2-methoxyethyl)-Pthioadenylyl-(3' 5')-2'-O-(2-methoxyethyl)-5-methyl-Pthiouridylyl-(3' 5')-2'-O-(2-methoxyethyl)-5-methyl-Pthiouridylyl-(3' 5')-2'-O-(2-methoxyethyl)-5-methylcytidine growth hormone receptor (GHR) expression inhibitor tout-p-ambo-2'-o-(2-méthoxyéthyl)-5-méthyl-p-thiouridylyl- (3' 5')-2'-O-(2-méthoxyéthyl)-5-méthyl-P-thiocytidylyl- (3' 5')-2'-O-(2-méthoxyéthyl)-P-thioadénylyl-(3' 5')-2'-O- (2-méthoxyéthyl)-P-thioguanylyl-(3' 5')-2'-O-(2- méthoxyéthyl)-p-thioguanylyl-(3' 5')-2'-désoxy-P- thioguanylyl-(3' 5')-2'-désoxy-5-méthyl-P-thiocytidylyl- (3' 5')-2'-désoxy-P-thioadénylyl-(3' 5')-P-thiothymidylyl- (3' 5')-P-thiothymidylyl-(3' 5')-2'-désoxy-5-méthyl-Pthiocytidylyl-(3' 5')-P-thiothymidylyl-(3' 5')-Pthiothymidylyl-(3' 5')-P-thiothymidylyl-(3' 5')-2'-désoxy-5- méthyl-p-thiocytidylyl-(3' 5')-2'-O-(2-méthoxyéthyl)-5- méthyl-p-thiocytidylyl-(3' 5')-2'-O-(2-méthoxyéthyl)-Pthioadénylyl-(3' 5')-2'-O-(2-méthoxyéthyl)-5-méthyl-Pthiouridylyl-(3' 5')-2'-O-(2-méthoxyéthyl)-5-méthyl-Pthiouridylyl-(3' 5')-2'-O-(2-méthoxyéthyl)-5-méthylcytidine inhibiteur de l'expression du récepteur de l'hormone de croissance 608
5 WHO Drug Information, Vol. 30, No. 4, 2016 Proposed INN: List 116 atesidorsén todo-p-ambo-2'-o-(2-metoxietil)-5-metil-p-tiouridilil-(3' 5')- 2'-O-(2-metoxietil)-5-metil-P-tiocitidilil-(3' 5')-2'-O-(2- metoxietil)-p-tioadenilil-(3' 5')-2'-O-(2-metoxietil)-P- tioguanilil-(3' 5')-2'-O-(2-metoxietil)-P-tioguanilil-(3' 5')- 2'-desoxi-P-tioguanilil-(3' 5')-2'-desoxi-5-metil-P-tiocitidilil- (3' 5')-2'-desoxi-P-tioadenilil-(3' 5')-P-tiotimidilil-(3' 5')- P-tiotimidilil-(3' 5')-2'-desoxi-5-metil-P-tiocitidilil-(3' 5')-P- tiotimidilil-(3' 5')-P-tiotimidilil-(3' 5')-P-tiotimidilil-(3' 5')- 2'-desoxi-5-metil-P-tiocitidilil-(3' 5')-2'-O-(2-metoxietil)-5- metil-p-tiocitidilil-(3' 5')-2'-O-(2-metoxietil)-P-tioadenilil- (3' 5')-2'-O-(2-metoxietil)-5-metil-P-tiouridilil-(3' 5')-2'-O- (2-metoxietil)-5-metil-P-tiouridilil-(3' 5')-2'-O-(2-metoxietil)- 5-metilcitidina inhibidor de la expresión del receptor de la hormona del crecimiento C 230 H 321 N 64 O 124 P 19 S (3'-5')(P-thio)(m 5 Umoe-m 5 Cmoe-Amoe-Gmoe-Gmoe-dG-m 5 dc-da-dt-dt-m 5 dc-dt-dt-dt-m 5 dc -m 5 Cmoe-Amoe-m 5 Umoe-m 5 Umoe-mCmoe) Legend: d (as prefix) = 2'-deoxy m 5 (as prefix) = 5-methyl moe (as suffix) = 2'-O-(2-methoxyethyl) atogepantum atogepant atogépant atogepant (3'S)-N-[(3S,5S,6R)-6-methyl-2-oxo-1-(2,2,2-trifluoroethyl)- 5-(2,3,6-trifluorophenyl)piperidin-3-yl]-2'-oxo-1',2',5,7- tetrahydrospiro[cyclopenta[b]pyridine-6,3'-pyrrolo[2,3- b]pyridine]-3-carboxamide calcitonin gene-related peptide receptor antagonist (3'S)-N-[(3S,5S,6R)-6-méthyl-2-oxo-1-(2,2,2-trifluoroéthyl)- 5-(2,3,6-trifluorophényl)pipéridin-3-yl]-2'-oxo-1',2',5,7- tétrahydrospiro[cyclopenta[b]pyridine-6,3'-pyrrolo[2,3- b]pyridine]-3-carboxamide antagoniste du récepteur du peptide lié au gène de la calcitonine (CGRP) (3'S)-N-[(3S,5S,6R)-6-metil-2-oxo-1-(2,2,2-trifluoroetil)- 5-(2,3,6-trifluorofenil)piperidin-3-il]-2'-oxo-1',2',5,7- tetrahidrospiro[ciclopenta[b]piridina-6,3'-pirrolo[2,3- b]piridina]-3-carboxamida antagonista del receptor del péptido relacionado con el gen de la calcitonina (CGRP) C 29 H 23 F 6 N 5 O
6 Proposed INN: List 116 WHO Drug Information, Vol. 30, No. 4, 2016 azeloprazolum azeloprazole azéloprazole azeloprazol 2-[(R)-{4-[(2,2-dimethyl-1,3-dioxan-5-yl)methoxy]-3,5- dimethylpyridin-2-yl}methanesulfinyl]-1h-benzimidazole proton pump inhibitor 2-[(R)-{4-[(2,2-diméthyl-1,3-dioxan-5-yl)méthoxy]-3,5- diméthylpyridin-2-yl}méthanesulfinyl]-1h-benzimidazole inhibiteur de la pompe à protons 2-[(R)-{4-[(2,2-dimetil-1,3-dioxan-5-il)metoxi]-3,5- dimetilpiridin-2-il}metanosulfinil]-1h-benzoimidazol inhibidor de la bomba de protones C 22 H 27 N 3 O 4 S azintuxizumabum # azintuxizumab azintuxizumab immunoglobulin G1-kappa, anti-[homo sapiens SLAMF7 (SLAM family member 7, CD2 subset 1, CS1, CD2-like receptor-activating cytotoxic cells, CRACC, 19A24, CD319)], humanized and chimeric monoclonal antibody; gamma1 heavy chain (1-447) [humanized VH (Homo sapiens IGHV3-7*01 (91.80%) -(IGHD) -IGHJ4*01 L123>T (112) [8.8.10] (1-117) -Homo sapiens IGHG1*03v, G1m3>G1m17, ng1m1 (CH1 R120>K (214) ( ), hinge ( ), CH2 ( ), CH3 E12(366), M14 (368) ( ), CHS ( ) ( )], ( ')- disulfide with kappa light chain chimeric (1'-220') [Mus musculus V-KAPPA (IGKV1-110*01 (93.00%) -IGKJ4*01) [ ] (1'-113') -Homo sapiens IGKC*01, Km3 A45.1 (159), V101 (197) (114'-220')]; dimer ( '': '')- bisdisulfide immunomodulator, antineoplastic immunoglobuline G1-kappa, anti-[homo sapiens SLAMF7 (membre 7 de la famille SLAM, CD2 subset 1, CS1, récepteur de type CD2 activant les cellules cytotoxiques, CRACC, 19A24, CD319)], anticorps monoclonal humanisé et chimérique; chaîne lourde gamma1 chain (1-447) [VH humanisé (Homo sapiens IGHV3-7*01 (91.80%) -(IGHD) -IGHJ4*01 L123>T (112)) [8.8.10] (1-117) -Homo sapiens IGHG1*03v, G1m3>G1m17, ng1m1 (CH1 R120>K (214) ( ), charnière ( ), CH2 ( ), CH3 E12(366), M14 (368) ( ), CHS ( ) ( )], ( ')- disulfure avec la chaîne légère kappa chimérique (1'-220') [V-KAPPA Mus musculus (IGKV1-110*01 (93.00%) - IGKJ4*01) [ ] (1'-113') -Homo sapiens IGKC*01, Km3 A45.1 (159), V101 (197) (114'-220')]; dimère ( '': '')-bisdisulfure immunomodulateur, antinéoplasique 610
7 WHO Drug Information, Vol. 30, No. 4, 2016 Proposed INN: List 116 azintuxizumab inmunoglobulina G1-kappa, anti-[homo sapiens SLAMF7 (miembro 7 de la familia SLAM, CD2 subset 1, CS1, receptor de tipo CD2 que activa las células citotóxicas, CRACC, 19A24, CD319)], anticuerpo monoclonal humanizado y quimérico; cadena pesada gamma1 cadena (1-447) [VH humanizado (Homo sapiens IGHV3-7*01 (91.80%) -(IGHD) -IGHJ4*01 L123>T (112)) [8.8.10] (1-117) -Homo sapiens IGHG1*03v, G1m3>G1m17, ng1m1 (CH1 R120>K (214) ( ), bisagra ( ), CH2 ( ), CH3 E12(366), M14 (368) ( ), CHS ( ) ( )], ( ')- disulfuro con la cadena ligera kappa quimérica (1'-220') [V-KAPPA Mus musculus (IGKV1-110*01 (93.00%) - IGKJ4*01) [ ] (1'-113') -Homo sapiens IGKC*01, Km3 A45.1 (159), V101 (197) (114'-220')]; dímero ( '': '')-bisdisulfuro inmunomodulador, antineoplásico Heavy chain / Chaîne lourde /Cadena pesada EVQLVESGGG LVQPGGSLRL SCAASGFTFS DYYMAWVRQA PGKGLEWVAS 50 INYDGSSTYY VDSVKGRFTI SRDNAKNSLY LQMNSLRAED TAVYYCARDR 100 GYYFDYWGQG TTVTVSSAST KGPSVFPLAP SSKSTSGGTA ALGCLVKDYF 150 PEPVTVSWNS GALTSGVHTF PAVLQSSGLY SLSSVVTVPS SSLGTQTYIC 200 NVNHKPSNTK VDKKVEPKSC DKTHTCPPCP APELLGGPSV FLFPPKPKDT 250 LMISRTPEVT CVVVDVSHED PEVKFNWYVD GVEVHNAKTK PREEQYNSTY 300 RVVSVLTVLH QDWLNGKEYK CKVSNKALPA PIEKTISKAK GQPREPQVYT 350 LPPSREEMTK NQVSLTCLVK GFYPSDIAVE WESNGQPENN YKTTPPVLDS 400 DGSFFLYSKL TVDKSRWQQG NVFSCSVMHE ALHNHYTQKS LSLSPGK 447 Light chain / Chaîne légère / Cadena ligera DVVMTQTPLS LSVTPGQPAS ISCRSSQSLV HSNGNTYLHW YLQKPGQSPQ 50 LLIYKVSNRF SGVPDRFSGS GSGTDFTLKI SRVEAEDVGV YFCSQSTHVP 100 PFTFGGGTKV EIKRTVAAPS VFIFPPSDEQ LKSGTASVVC LLNNFYPREA 150 KVQWKVDNAL QSGNSQESVT EQDSKDSTYS LSSTLTLSKA DYEKHKVYAC 200 EVTHQGLSSP VTKSFNRGEC 220 Post-translational modifications Disulfide bridges location / Position des ponts disulfure / Posiciones de los puentes disulfuro Intra-H (C23-C104) ''-96'' 144''-200'' 261''-321'' 367''-425'' Intra-L (C23-C104) 23'-93' 140'-200' 23'''-93''' 140'''-200''' Inter-H-L (h 5-CL 126) ' 220''-220''' Inter-H-H (h 11, h 14) '' '' N-glycosylation sites / Sites de N-glycosylation / Posiciones de N-glicosilación H CH2 N84.4: 297, 297'' Fucosylated complex bi-antennary CHO-type glycans (G0F, G1F) / glycanes de type CHO bi-antennaires complexes fucosylés (G0F, G1F) / glicanos de tipo CHO biantenarios complejos fucosilados (G0F, G1F) azintuxizumabum vedotinum # azintuxizumab vedotin immunoglobulin G1-kappa, anti-[homo sapiens SLAMF7 (SLAM family member 7, CD2 subset 1, CS1, CD2-like receptor-activating cytotoxic cells, CRACC, 19A24, CD319)], humanized and chimeric monoclonal antibody antibody conjugated to auristatin E; gamma1 heavy chain (1-447) [humanized VH (Homo sapiens IGHV3-7*01(91.80%) -(IGHD) -IGHJ4*01 L123>T (112)) [8.8.10] (1-117) -Homo sapiens IGHG1*03v, G1m3>G1m17, ng1m1 (CH1 R120>K (214) ( ), hinge ( ), CH2 ( ), CH3 E12(366), M14 (368) ( ), CHS ( ) ( )], ( ')- 611
8 Proposed INN: List 116 WHO Drug Information, Vol. 30, No. 4, 2016 disulfide with kappa light chain chimeric (1'-220') [Mus musculus V-KAPPA (IGKV1-110*01 (93.00%) -IGKJ4*01) [ ] (1'-113') -Homo sapiens IGKC*01, Km3 A45.1 (159), V101 (197) (114'-220')]; dimer ( '': '')- bisdisulfide; conjugated, on an average of 3 cysteinyl, to monomethylauristatin E (MMAE), via a cleavable maleimidocaproyl-valyl-citrullinyl-paminobenzyloxycarbonyl (mc-val-cit-pabc) type linker For the vedotin part, please refer to the document "INN for pharmaceutical substances: Names for radicals, groups and others"*. immunomodulator, antineoplastic azintuxizumab védotine azintuxizumab vedotina immunoglobuline G1-kappa, anti-[homo sapiens SLAMF7 (membre 7 de la famille SLAM, CD2 subset 1, CS1, récepteur de type CD2 activant les cellules cytotoxiques, CRACC, 19A24, CD319)], anticorps monoclonal humanisé et chimérique conjugué à l'auristatine E; chaîne lourde gamma1 (1-447) [VH humanisé (Homo sapiens IGHV3-7*01(91.80%) -(IGHD) -IGHJ4*01 L123>T (112)) [8.8.10] (1-117) -Homo sapiens IGHG1*03v, G1m3>G1m17, ng1m1 (CH1 R120>K (214) ( ), charnière ( ), CH2 ( ), CH3 E12(366), M14 (368) ( ), CHS ( ) ( )], ( ')- disulfure avec la chaîne légère kappa chimérique (1'-220') [V-KAPPA Mus musculus (IGKV1-110*01 (93.00%) - IGKJ4*01) [ ] (1'-113') -Homo sapiens IGKC*01, Km3 A45.1 (159), V101 (197) (114'-220')]; dimère ( '': '')-bisdisulfure; conjugué, sur 3 cystéinyl en moyenne, au monométhylauristatine E (MMAE), via un linker clivable de type maléimidocaproyl-valyl-citrullinyl-paminobenzyloxycarbonyl (mc-val-cit-pabc) Pour la partie védotine, veuillez-vous référer au document "INN for pharmaceutical substances: Names for radicals, groups and others"*. immunomodulateur, antinéoplasique inmunoglobulina G1-kappa, anti-[homo sapiens SLAMF7 (miembro 7 de la familia SLAM, CD2 subset 1, CS1, receptor de tipo CD2 que activa las células citotóxicas, CRACC, 19A24, CD319)], anticuerpo monoclonal humanizado y quimérico conjugado con la auristatina E; cadena pesada gamma1 (1-447) [VH humanizado (Homo sapiens IGHV3-7*01(91.80%) -(IGHD) -IGHJ4*01 L123>T (112)) [8.8.10] (1-117) -Homo sapiens IGHG1*03v, G1m3>G1m17, ng1m1 (CH1 R120>K (214) ( ), bisagra ( ), CH2 ( ), CH3 E12(366), M14 (368) ( ), CHS ( ) ( )], ( ')- disulfuro con la cadena ligera kappa quimérica (1'-220') [V- KAPPA Mus musculus (IGKV1-110*01 (93.00%) - IGKJ4*01) [ ] (1'-113') -Homo sapiens IGKC*01, Km3 A45.1 (159), V101 (197) (114'-220')]; dímero ( '': '')-bisdisulfuro; conjugado, en 3 residuos cisteinil por término medio, con monometilauristatina E (MMAE), con un conector de type maleimidocaproil-valilcitrulinil-p-aminobenziloxicarbonil (mc-val-cit-pabc) escindible Para la fracción védotine, se pueden referir al documento "INN for pharmaceutical substances: Names for radicals, groups and others"*. inmunomodulador, antineoplásico 612
9 WHO Drug Information, Vol. 30, No. 4, 2016 Proposed INN: List Heavy chain / Chaîne lourde /Cadena pesada EVQLVESGGG LVQPGGSLRL SCAASGFTFS DYYMAWVRQA PGKGLEWVAS 50 INYDGSSTYY VDSVKGRFTI SRDNAKNSLY LQMNSLRAED TAVYYCARDR 100 GYYFDYWGQG TTVTVSSAST KGPSVFPLAP SSKSTSGGTA ALGCLVKDYF 150 PEPVTVSWNS GALTSGVHTF PAVLQSSGLY SLSSVVTVPS SSLGTQTYIC 200 NVNHKPSNTK VDKKVEPKSC DKTHTCPPCP APELLGGPSV FLFPPKPKDT 250 LMISRTPEVT CVVVDVSHED PEVKFNWYVD GVEVHNAKTK PREEQYNSTY 300 RVVSVLTVLH QDWLNGKEYK CKVSNKALPA PIEKTISKAK GQPREPQVYT 350 LPPSREEMTK NQVSLTCLVK GFYPSDIAVE WESNGQPENN YKTTPPVLDS 400 DGSFFLYSKL TVDKSRWQQG NVFSCSVMHE ALHNHYTQKS LSLSPGK 447 Light chain / Chaîne légère / Cadena ligera DVVMTQTPLS LSVTPGQPAS ISCRSSQSLV HSNGNTYLHW YLQKPGQSPQ 50 LLIYKVSNRF SGVPDRFSGS GSGTDFTLKI SRVEAEDVGV YFCSQSTHVP 100 PFTFGGGTKV EIKRTVAAPS VFIFPPSDEQ LKSGTASVVC LLNNFYPREA 150 KVQWKVDNAL QSGNSQESVT EQDSKDSTYS LSSTLTLSKA DYEKHKVYAC 200 EVTHQGLSSP VTKSFNRGEC 220 Post-translational modifications Disulfide bridges location / Position des ponts disulfure / Posiciones de los puentes disulfuro Intra-H (C23-C104) ''-96'' 144''-200'' 261''-321'' 367''-425'' Intra-L (C23-C104) 23'-93' 140'-200' 23'''-93''' 140'''-200''' Inter-H-L (h 5-CL 126)* ' 220''-220''' Inter-H-H (h 11, h 14)* '' '' *Two or three of the inter-chain disulfide bridges are not present, an average of 3 cysteinyl being conjugated each via a thioether bond to a drug linker. *Deux ou trois des ponts disulfures inter-chaînes ne sont pas présents, 3 cystéinyl en moyenne étant chacun conjugué via une liaison thioéther à un linker-principe actif. *Faltan dos o tres puentes disulfuro inter-catenarios, una media de 3 cisteinil está conjugada a conectores de principio activo. N-glycosylation sites / Sites de N-glycosylation / Posiciones de N-glicosilación H CH2 N84.4: 297, 297'' Fucosylated complex bi-antennary CHO-type glycans (G0F, G1F) / glycanes de type CHO bi-antennaires complexes fucosylés (G0F, G1F) / glicanos de tipo CHO biantenarios complejos fucosilados (G0F, G1F) baliforsenum baliforsen baliforsen all-p-ambo-2'-o-(2-methoxyethyl)-5-methyl-p-thiouridylyl- (3' 5')-2'-O-(2-methoxyethyl)-5-methyl-P-thiocytidylyl- (3' 5')-2'-O,4'-C-[(1S)-ethane-1,1-diyl]-5-methyl-P- thiocytidylyl-(3' 5')-2'-O,4'-C-[(1S)-ethane-1,1-diyl]-5- methyl-p-thiocytidylyl-(3' 5')-2'-deoxy-P-thioguanylyl- (3' 5')-2'-deoxy-P-thioadenylyl-(3' 5')-2'-deoxy-Pthioadenylyl-(3' 5')-P-thiothymidylyl-(3' 5')-2'-deoxy-Pthioguanylyl-(3' 5')-P-thiothymidylyl-(3' 5')-2'-deoxy-5- methyl-p-thiocytidylyl-(3' 5')-2'-deoxy-5-methyl-Pthiocytidylyl-(3' 5')-2'-O,4'-C-[(1S)-ethane-1,1-diyl]-Pthioguanylyl-(3' 5')-2'-O,4'-C-[(1S)-ethane-1,1-diyl]-Pthioadenylyl-(3' 5')-2'-O-(2-methoxyethyl)-5-methyl-Pthiocytidylyl-(3' 5')-2'-O-(2-methoxyethyl)adenosine dystrophia myotonica-protein kinase (DMPK) synthesis inhibitor tout-p-ambo-2'-o-(2-méthoxyéthyl)-5-méthyl-p-thiouridylyl- (3' 5')-2'-O-(2-méthoxyéthyl)-5-méthyl-P-thiocytidylyl- (3' 5')-2'-O,4'-C-[(1S)-éthane-1,1-diyl]-5-méthyl-P- thiocytidylyl-(3' 5')-2'-O,4'-C-[(1S)-éthane-1,1-diyl]-5- méthyl-p-thiocytidylyl-(3' 5')-2'-désoxy-P-thioguanylyl- (3' 5')-2'-désoxy-P-thioadénylyl-(3' 5')-2'-désoxy-Pthioadénylyl-(3' 5')-P-thiothymidylyl-(3' 5')-2'-désoxy-Pthioguanylyl-(3' 5')-P-thiothymidylyl-(3' 5')-2'-désoxy-5- méthyl-p-thiocytidylyl-(3' 5')-2'-désoxy-5-méthyl-Pthiocytidylyl-(3' 5')-2'-O,4'-C-[(1S)-éthane-1,1-diyl]-Pthioguanylyl-(3' 5')-2'-O,4'-C-[(1S)-éthane-1,1-diyl]-Pthioadénylyl-(3' 5')-2'-O-(2-méthoxyéthyl)-5-méthyl-Pthiocytidylyl-(3' 5')-2'-O-(2-méthoxyéthyl)adénosine inhibiteur de la synthèse de la protéine-kinase de la dystrophie myotonique (DMPK) 613
10 Proposed INN: List 116 WHO Drug Information, Vol. 30, No. 4, 2016 baliforsén todo-p-ambo-2'-o-(2-metoxietil)-5-metil-p-tiouridilil-(3' 5')- 2'-O-(2-metoxietil)-5-metil-P-tiocitidilil-(3' 5')-2'-O,4'-C- [(1S)-etano-1,1-diil]-5-metil-P-tiocitidilil-(3' 5')-2'-O,4'-C- [(1S)-etano-1,1-diil]-5-metil-P-tiocitidilil-(3' 5')-2'-desoxi-P- tioguanilil-(3' 5')-2'-desoxi-P-tioadenilil-(3' 5')-2'-desoxi- P-tioadenilil-(3' 5')-P-tiotimidilil-(3' 5')-2'-desoxi-Ptioguanilil-(3' 5')-P-tiotimidilil-(3' 5')-2'-desoxi-5-metil-Ptiocitidilil-(3' 5')-2'-desoxi-5-metil-P-tiocitidilil-(3' 5')-2'- O,4'-C-[(1S)-etano-1,1-diil]-P-tioguanilil-(3' 5')-2'-O,4'-C- [(1S)-etano-1,1-diil]-P-tioadenilil-(3' 5')-2'-O-(2-metoxietil)- 5-metil-P-tiocitidilil-(3' 5')-2'-O-(2-metoxietil)adenosina inhibidor de la síntesis de la proteína-kinasa de la distrofia miotónica (DMPK) C 180 H 240 N 59 O 90 P 15 S (3'-5')-(P-thio)(m5Umoe-m5Cmoe-m5C(Et)-m5C(Et)-dG-dA-dA-dT-dG-dT-m5dC-m5dC-G(Et)-A(Et)- m5cmoe-amoe) Legend: d as prefix = 2'-deoxy (Et) as suffix = 2'-O,4'-C-[(1S)-ethane-1,1-diyl]* m5 as prefix = 5-methyl moe as suffix = 2'-O-(2-methoxyethyl) OH X O N H 3C H * (Et) as suffix HO O balipodectum balipodect balipodect balipodect 1-[2-fluoro-4-(1H-pyrazol-1-yl)phenyl]-5-methoxy- 3-(1-phenyl-1H-pyrazol-5-yl)pyridazin-4(1H)-one antipsychotic 1-[2-fluoro-4-(1H-pyrazol-1-yl)phényl]-5-méthoxy- 3-(1-phényl-1H-pyrazol-5-yl)pyridazin-4(1H)-one antipsychotique 1-[2-fluoro-4-(1H-pirazol-1-il)fenil]-5-metoxi-3-(1-fenil- 1H-pirazol-5-il)piridazin-4(1H)-ona antipsicótico C 23 H 17 FN 6 O balovaptanum balovaptan balovaptan 8-chloro-5-methyl-1-{trans-4-[(pyridin-2-yl)oxy]cyclohexyl}- 5,6-dihydro-4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine vasopressin receptor antagonist 8-chloro-5-méthyl-1-{trans-4-[(pyridin-2-yl)oxy]cyclohexyl}- 5,6-dihydro-4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazépine antagoniste du récepteur de la vasopressine 614
11 WHO Drug Information, Vol. 30, No. 4, 2016 Proposed INN: List 116 balovaptán 8-cloro-5-metil-1-{trans-4-[(piridin-2-il)oxi]ciclohexil}- 5,6-dihidro-4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepina antagonista del receptor de la vasopresina C 22 H 24 ClN 5 O N O N N N Cl N CH 3 baloxavirum marboxilum baloxavir marboxil baloxavir marboxil baloxavir marboxil ({(12aR)-12-[(11S)-7,8-difluoro-6,11- dihydrodibenzo[b,e]thiepin-11-yl]-6,8-dioxo-3,4,6,8,12,12a- hexahydro-1h-[1,4]oxazino[3,4-c]pyrido[2,1-f][1,2,4]triazin- 7-yl}oxy)methyl methyl carbonate antiviral carbonate de({(12ar)-12-[(11s)-7,8-difluoro-6,11- dihydrodibenzo[b,e]thiépin-11-yl]-6,8-dioxo-3,4,6,8,12,12a- hexahydro-1h-[1,4]oxazino[3,4-c]pyrido[2,1-f][1,2,4]triazin- 7-yl}oxy)méthyle et de méthyle antiviral carbonato de({(12ar)-12-[(11s)-7,8-difluoro-6,11- dihidrodibenzo[b,e]tiepin-11-il]-6,8-dioxo-3,4,6,8,12,12a- hexahidro-1h-[1,4]oxazino[3,4-c]pirido[2,1-f][1,2,4]triazin- 7-il}oxi)metilo y de metilo antiviral C 27 H 23 F 2 N 3 O 7 S baltaleucelum baltaleucel Autologous Epstein-Barr virus (EBV)-specific T cells derived from peripheral blood mononuclear cells (PBMCs) stimulated and expanded for enrichment of CD4+ and CD8+ memory and effector T cells with specificity for a range of epitopes across four EBV antigens (latent membrane protein 1 (LMP1), latent membrane protein 2 (LMP2), EBV nuclear antigen 1 (EBNA1), and BamHI-A rightward frame 1 (BARF1)). Contains CD3 + T cells, CD3 - CD16 + CD56 + natural killer (NK) cells and CD3 + CD56 + natural killer T (NKT) cells in proportions varying per individual patient. cell therapy (antineoplastic) 615
12 Proposed INN: List 116 WHO Drug Information, Vol. 30, No. 4, 2016 baltaleucel baltaleucel Lymphocytes T autologues spécifiques du virus d'epstein- Barr (EBV) dérivés de cellules mononucléaires du sang périphérique (PMBCs), stimulés et expansés pour enrichissement des lymphocytes T mémoire CD4+ et CD8+ ayant une spécificité vis-à-vis d'un éventail d'épitopes de quatre antigènes d'ebv (protéine latente de membrane 1 (LMP1), protéine latente de membrane 2 (LMP2), antigène nucléaire de EBV 1 (EBNA1), et BARF1 (BamHI-A rightward frame 1)). contient des lymphocytes T CD3+, cellules tueuses naturelles (NK) CD3- CD16+ CD56+ et des lymphocytes T NK CD3+ CD56+ en proportions variables pour chaque patient thérapie cellulaire (antinéoplasique) Linfocitos T autólogos específicos frente al virus de Epstein-Barr (EBV) derivados de células mononucleares de sangre periférica (PBMCs), estimulados y expandidos para enriquecimiento de los linfocitos T CD4+ y CD8+ efectores y de memoria con especificidad para un rango de epítopos presentes a lo largo de cuatro antígenos de EBV (proteína latente de membrana 1 (LMP1), proteína latente de membrana 2 (LMP2), antígeno nuclear de EBV 1 (EBNA1), y BARF1). Contiene linfocitos T CD3+, células NK CD3- CD16+ CD56+ y linfocitos T NK CD3+ CD56+ en proporciones variables para cada paciente individual. terapia celular (antineoplásico) benzodrocortisonum benzodrocortisone benzodrocortisone benzodrocortisona 11β,21-dihydroxy-3,20-dioxopregn-4-en-17-yl benzoate corticosteroid benzoate de 11β,21-dihydroxy-3,20-dioxoprégn-4-én- 17-yle corticostéroïde benzoato de 11β,21-dihidroxi-3,20-dioxopregn-4-en-17-ilo corticosteroide C 28 H 34 O OH O H CH 3 HO O CH 3 H O H H O betibeglogenum darolentivecum # betibeglogene darolentivec A self-inactivating human immunodeficiency virus-1 (HIV- 1)-derived lentiviral vector encoding a T87Q-mutated form of the human hemoglobin subunit beta (HBB, beta-globin) gene under the control of a human β-globin promoter and a 3' β-globin enhancer gene therapy (beta-thalassemia) 616
13 WHO Drug Information, Vol. 30, No. 4, 2016 Proposed INN: List 116 bétibéglogène darolentivec betibeglogén darolentivec Vecteur lentiviral auto-inactivant dérivé du virus de l immunodéficience humaine-1 (HIV-1) codant pour une forme mutée (T87Q) du gène de la sous-unité bêta de l hémoglobine humaine (HBB, bêta-globine) sous le contrôle d un promoteur de la β-globine humaine et un activateur de la β-globine en position 3. thérapie génique (bêta-thalassémie) Un vector lentiviral auto-inactivante derivado del virus de la inmunodeficiencia humana 1 (VIH-1) que contiene el gen que codifica para una forma mutada (T87Q) de la subunidad beta de la hemoglobina humana (HBB, betaglobina) bajo el control de un promotor de la β-globina humana y un potenciador (enhancer) de la β-globina en posición 3. terapia génica (beta-talasemia) bimiralisibum bimiralisib bimiralisib bimiralisib 5-[4,6-di(morpholin-4-yl)-1,3,5-triazin-2-yl]- 4-(trifluoromethyl)pyridin-2-amine antineoplastic 5-[4,6-di(morpholin-4-yl)-1,3,5-triazin-2-yl]- 4-(trifluorométhyl)pyridin-2-amine antinéoplasique 5-[4,6-di(morfolin-4-il)-1,3,5-triazin-2-il]- 4-(trifluorometil)piridin-2-amino antineoplásico C 17 H 20 F 3 N 7 O brivoligidum brivoligide 2'-deoxycytidylyl-(3' 5')-thymidylyl-(5' 3')-2'- deoxyadenylyl-(3' 5')-2'-deoxycytidylyl-(3' 5')-2'- deoxyguanylyl-(3' 5')-2'-deoxycytidylyl-(3' 5')-2'- deoxycytidylyl-(3' 5')-2'-deoxycytidylyl-(3' 5')-2'- deoxyadenylyl-(3' 5')-2'-deoxycytidylyl-(3' 5')-2'- deoxycytidylyl-(3' 5')-2'-deoxyguanylyl-(3' 5')-2'- deoxycytidylyl-(3' 5')-2'-deoxycytidylyl-(3' 5')-2'- deoxycytidylyl-(3' 5')-2'-deoxyadenylyl-(3' 5')-2'- deoxycytidylyl-(3' 5')-2'-deoxyguanylyl-(3' 5')-2'- deoxycytidylyl-(3' 5')-2'-deoxyadenylyl-(3' 5')-thymidylyl- (5' 3')-2'-deoxyadenylyl-(3' 5')-2'-deoxycytidine duplex 617
14 Proposed INN: List 116 WHO Drug Information, Vol. 30, No. 4, 2016 with 2'-deoxyguanylyl-(5' 3')-2'-deoxyadenylyl-(5' 3')- thymidylyl-(5' 3')-2'-deoxyguanylyl-(5' 3')-2'- deoxycytidylyl-(5' 3')-2'-deoxyguanylyl-(5' 3')-2'- deoxyguanylyl-(5' 3')-2'-deoxyguanylyl-(5' 3')-thymidylyl- (5' 3')-2'-deoxyguanylyl-(5' 3')-2'-deoxyguanylyl-(5' 3')- 2'-deoxycytidylyl-(5' 3')-2'-deoxyguanylyl-(5' 3')-2'- deoxyguanylyl-(5' 3')-2'-deoxyguanylyl-(5' 3')-thymidylyl- (5' 3')-2'-deoxyguanylyl-(5' 3')-2'-deoxycytidylyl-(5' 3')- 2'-deoxyguanylyl-(5' 3')-thymidylyl-(5' 3')-2'- deoxyadenylyl-(5' 3')-thymidylyl-(5' 3')-2'- deoxyguanosine analgesic brivoligide brivoligida 2'-désoxycytidylyl-(3' 5')-thymidylyl-(5' 3')-2'- désoxyadénylyl-(3' 5')-2'-désoxycytidylyl-(3' 5')-2'- désoxyguanylyl-(3' 5')-2'-désoxycytidylyl-(3' 5')-2'- désoxycytidylyl-(3' 5')-2'-désoxycytidylyl-(3' 5')-2'- désoxyadénylyl-(3' 5')-2'-désoxycytidylyl-(3' 5')-2'- désoxycytidylyl-(3' 5')-2'-désoxyguanylyl-(3' 5')-2'- désoxycytidylyl-(3' 5')-2'-désoxycytidylyl-(3' 5')-2'- désoxycytidylyl-(3' 5')-2'-désoxyadénylyl-(3' 5')-2'- désoxycytidylyl-(3' 5')-2'-désoxyguanylyl-(3' 5')-2'- désoxycytidylyl-(3' 5')-2'-désoxyadénylyl-(3' 5')- thymidylyl-(5' 3')-2'-désoxyadénylyl-(3' 5')-2'- désoxycytidine duplex avec 2'-désoxyguanylyl-(5' 3')-2'- désoxyadénylyl-(5' 3')-thymidylyl-(5' 3')-2'- désoxyguanylyl-(5' 3')-2'-désoxycytidylyl-(5' 3')-2'- désoxyguanylyl-(5' 3')-2'-désoxyguanylyl-(5' 3')-2'- désoxyguanylyl-(5' 3')-thymidylyl-(5' 3')-2'- désoxyguanylyl-(5' 3')-2'-désoxyguanylyl-(5' 3')-2'- désoxycytidylyl-(5' 3')-2'-désoxyguanylyl-(5' 3')-2'- désoxyguanylyl-(5' 3')-2'-désoxyguanylyl-(5' 3')- thymidylyl-(5' 3')-2'-désoxyguanylyl-(5' 3')-2'- désoxycytidylyl-(5' 3')-2'-désoxyguanylyl-(5' 3')- thymidylyl-(5' 3')-2'-désoxyadénylyl-(5' 3')-thymidylyl- (5' 3')-2'-désoxyguanosine analgésique 2'-desoxicitidilil-(3' 5')-timidilil-(5' 3')-2'-desoxiadenilil- (3' 5')-2'-desoxicitidilil-(3' 5')-2'-desoxiguanilil-(3' 5')-2'- desoxicitidilil-(3' 5')-2'-desoxicitidilil-(3' 5')-2'- desoxicitidilil-(3' 5')-2'-desoxiadenilil-(3' 5')-2'- desoxicitidilil-(3' 5')-2'-desoxicitidilil-(3' 5')-2'- desoxiguanilil-(3' 5')-2'-desoxicitidilil-(3' 5')-2'- desoxicitidilil-(3' 5')-2'-desoxicitidilil-(3' 5')-2'- desoxiadenilil-(3' 5')-2'-desoxicitidilil-(3' 5')-2'- desoxiguanilil-(3' 5')-2'-desoxicitidilil-(3' 5')-2'- desoxiadenilil-(3' 5')-timidilil-(5' 3')-2'-desoxiadenilil- (3' 5')-2'-desoxicitidina dúplex con 2'-desoxiguanilil- (5' 3')-2'-desoxiadenilil-(5' 3')-timidilil-(5' 3')-2'- desoxiguanilil-(5' 3')-2'-desoxicitidilil-(5' 3')-2'- desoxiguanilil-(5' 3')-2'-desoxiguanilil-(5' 3')-2'- desoxiguanilil-(5' 3')-timidilil-(5' 3')-2'-desoxiguanilil- (5' 3')-2'-desoxiguanilil-(5' 3')-2'-desoxicitidilil-(5' 3')-2'- desoxiguanilil-(5' 3')-2'-desoxiguanilil-(5' 3')-2'- desoxiguanilil-(5' 3')-timidilil-(5' 3')-2'-desoxiguanilil- (5' 3')-2'-desoxicitidilil-(5' 3')-2'-desoxiguanilil-(5' 3')- timidilil-(5' 3')-2'-desoxiadenilil-(5' 3')-timidilil-(5' 3')-2'- desoxiguanosina analgésico 618
15 WHO Drug Information, Vol. 30, No. 4, 2016 Proposed INN: List 116 C 444 H 561 N 177 O 272 P (3'-5') d(c-t-a-c-g-c-c-c-a-c-c-g-c-c-c-a-c-g-c-a-t-a-c) (5'-3') d(g-a-t-g-c-g-g-g-t-g-g-c-g-g-g-t-g-c-g-t-a-t-g) cavosonstatum cavosonstat cavosonstat cavosonstat 3-chloro-4-(6-hydroxyquinolin-2-yl)benzoic acid alcohol dehydrogenase inhibitor acide 3-chloro-4-(6-hydroxyquinoléin-2-yl)benzoïque inhibiteur de l'alcool déshydrogénase ácido 3-cloro-4-(6-hidroxiquinolein-2-il)benzoico inhibidor de la alcohol deshidrogenasa C 16 H 10 ClNO ceclazepidum ceclazepide céclazépide ceclazepida 2,2-dimethyl-4-[(3R)-3-({[3- (methylamino)phenyl]carbamoyl}amino)-2-oxo-5-(pyridin- 2-yl)-2,3-dihydro-1H-1,4-benzodiazepin-1-yl]-3-oxobutyl acetate cholecystokinin receptor antagonist acétate de 2,2-diméthyl-4-[(3R)-3-({[3- (méthylamino)phényl]carbamoyl}amino)-2-oxo-5-(pyridin- 2-yl)-2,3-dihydro-1H-1,4-benzodiazépin-1-yl]-3-oxobutyle antagoniste des récepteurs des cholécystokinines acetato de 2,2-dimetil-4-[(3R)-3-({[3- (metilamino)fenil]carbamoil}amino)-2-oxo-5-(piridin-2-il)- 2,3-dihidro-1H-1,4-benzodiazepin-1-il]-3-oxobutilo antagonista de los receptores de las colecistoquininas C 30 H 32 N 6 O citarinostatum citarinostat 2-(2-chloro-N-phenylanilino)-N-[7-(hydroxyamino)- 7-oxoheptyl]pyrimidine-5-carboxamide histone deacetylase inhibitor, antineoplastic 619
16 Proposed INN: List 116 WHO Drug Information, Vol. 30, No. 4, 2016 citarinostat citarinostat 2-(2-chloro-N-phénylanilino)-N-[7-(hydroxyamino)- 7-oxoheptyl]pyrimidine-5-carboxamide inhibiteur de l'histone désacétylase, antinéoplasique 2-(2-cloro-N-fenilanilina)-N-[7-(hidroxiamino)- 7-oxoheptil]pirimidina-5-carboxamida inhibidor de la histona desacetilasa, antineoplásico C 24 H 26 ClN 5 O N N Cl N O H N O H N OH cosdosiranum cosdosiran cosdosiran adenylyl-(3' 5')-2'-O-methylguanylyl-(3' 5')-guanylyl- (3' 5')-2'-O-methyladenylyl-(3' 5')-guanylyl-(3' 5')-2'-O- methyluridylyl-(3' 5')-uridylyl-(3' 5')-2'-O-methylcytidylyl- (3' 5')-cytidylyl-(3' 5')-adenylyl-(3' 5')-2'-Omethylcytidylyl-(3' 5')-adenylyl-(3' 5')-2'-Omethyluridylyl-(3' 5')-uridylyl-(3' 5')-2'-O-methylcytidylyl- (3' 5')-uridylyl-(3' 5')-2'-O-methylguanylyl-(3' 5')- guanylyl-(3' 5')-2'-O-methylcytidine duplex with [(2R,3S)- 3-hydroxyoxolan-2-yl]methyl hydrogen uridylyl-(5' 3')-2'- deoxycytidylyl-(5 3 )-cytidylyl-(5 3 )-uridylyl-(5' 3')- cytidylyl-(5 3 )-adenylyl-(5 3 )-adenylyl-(5 3 )- guanylyl-(5 3 )-guanylyl-(5 3 )-uridylyl-(5' 3')-guanylyl- (5 3 )-uridylyl-(5' 3')-adenylyl-(5 3 )-adenylyl-(5 3 )- guanylyl-(5 3 )-adenylyl-(5 3 )-cytidylyl-(5 3 )- cytidylyl-(5 3 )-5'-guanylate inhibition of caspase 2 synthesis adénylyl-(3' 5')-2'-O-méthylguanylyl-(3' 5')-guanylyl- (3' 5')-2'-O-méthyladénylyl-(3' 5')-guanylyl-(3' 5')-2'-O- méthyluridylyl-(3' 5')-uridylyl-(3' 5')-2'-O-méthylcytidylyl- (3' 5')-cytidylyl-(3' 5')-adénylyl-(3' 5')-2'-Ométhylcytidylyl-(3' 5')-adénylyl-(3' 5')-2'-Ométhyluridylyl-(3' 5')-uridylyl-(3' 5')-2'-O-méthylcytidylyl- (3' 5')-uridylyl-(3' 5')-2'-O-méthylguanylyl-(3' 5')- guanylyl-(3' 5')-2'-O-méthylcytidine duplex avec l uridylyl- (5' 3')-2'-désoxycytidylyl-(5 3 )-cytidylyl-(5 3 )-uridylyl- (5' 3')-cytidylyl-(5 3 )-adénylyl-(5 3 )-adénylyl-(5 3 )- guanylyl-(5 3 )-guanylyl-(5 3 )-uridylyl-(5' 3')-guanylyl- (5 3 )-uridylyl-(5' 3')-adénylyl-(5 3 )-adénylyl-(5 3 )- guanylyl-(5 3 )-adénylyl-(5 3 )-cytidylyl-(5 3 )- cytidylyl-(5 3 )-hydrogéno-5'-guanylate de [(2R,3S)-3- hydroxyoxolan-2-yl]méthyle inhibition de la synthèse de la caspase 2 620
17 WHO Drug Information, Vol. 30, No. 4, 2016 Proposed INN: List 116 cosdosirán adenilil-(3' 5')-2'-O-metilguanilil-(3' 5')-guanilil-(3' 5')-2'- O-metiladenilil-(3' 5')-guanilil-(3' 5')-2'-O-metiluridilil- (3' 5')-uridilil-(3' 5')-2'-O-metilcitidilil-(3' 5')-citidilil- (3' 5')-adenilil-(3' 5')-2'-O-metilcitidilil-(3' 5')-adenilil- (3' 5')-2'-O-metiluridilil-(3' 5')-uridilil-(3' 5')-2'-O- metilcitidilil-(3' 5')-uridilil-(3' 5')-2'-O-metilguanilil- (3' 5')-guanilil-(3' 5')-2'-O-metilcitidina duplex con uridilil- (5' 3')-2'-desoxicitidilil-(5 3 )-citidilil-(5 3 )-uridilil- (5' 3')-citidilil-(5 3 )-adenilil-(5 3 )-adenilil-(5 3 )- guanilil-(5 3 )-guanilil-(5 3 )-uridilil-(5' 3')-guanilil- (5 3 )-uridilil-(5' 3')-adenilil-(5 3 )-adenilil-(5 3 )- guanilil-(5 3 )-adenilil-(5 3 )-citidilil-(5 3 )-citidilil- (5 3 )-hidrógeno -5'-guanilato de [(2R,3S)-3- hidroxioxolan-2-il]metilo inhibición de la síntesis de la caspasa 2 C 375 H 476 N 143 O 266 P (3' 5') A G G A G U U C C A C A U U C U G G C (5' 3') U C* C U C A A G G U G U A A G A C C G R Legend X : 2'-O-methyl (Xm) C * : 2'-deoxycytidylyl O OH R = O P O O HO cosfroviximabum # cosfroviximab cosfroviximab immunoglobulin G1-kappa, anti-[reston ebolavirus, Sudan ebolavirus,tai Forest ebolavirus, Zaire ebolavirus (Zaire Ebola virus (EBOV)) glycoprotein], chimeric monoclonal antibody; gamma1 heavy chain (1-452) [Mus musculus VH (IGHV8-8*01 (76.50%) -(IGHD) -IGHJ4*01) [ ] (1-122) - Homo sapiens IGHG1*01v, G1m17>G1m3, G1m1 (CH1 K120>R (219) ( ), hinge ( ), CH2 ( ), CH3 D12 (361), L14 (363) ( ), CHS ( )) ( )], ( ')-disulfide with kappa light chain (1'- 213') [Mus musculus V-KAPPA (IGKV6-13*01 (94.70%) - IGKJ5*01) [6.3.9] (1'-106') -Homo sapiens IGKC*01, Km3 A45.1 (152), V101 (190) (107'-213')]; dimer ( '': '')-bisdisulfide immunomodulator, antiviral immunoglobuline G1-kappa, anti-[glycoprotéine de Reston ebolavirus, Sudan ebolavirus,tai Forest ebolavirus, Zaire ebolavirus (virus Ebola Zaïre (EBOV))], anticorps monoclonal chimérique; chaîne lourde gamma1 (1-452) [Mus musculus VH (IGHV8-8*01(76.50%) -(IGHD) -IGHJ4*01) [ ] (1-122) -Homo sapiens IGHG1*01v, G1m17>G1m3, G1m1 (CH1 K120>R (219) ( ), charnière ( ), CH2 ( ), CH3 D12 (361), L14 (363) ( ), CHS ( )) ( )], ( ')-disulfure avec la chaîne légère kappa (1'-213') [Mus musculus V-KAPPA (IGKV6-13*01 (94.70%) -IGKJ5*01) [6.3.9] (1'-106') -Homo sapiens IGKC*01, Km3 A45.1 (152), V101 (190) (107'-213')]; dimère ( '': '')-bisdisulfure immunomodulateur, antiviral 621
18 Proposed INN: List 116 WHO Drug Information, Vol. 30, No. 4, 2016 cosfroviximab inmunoglobulina G1-kappa, anti-[glicoproteína de Reston ebolavirus, Sudan ebolavirus,tai Forest ebolavirus, Zaire ebolavirus (virus Ebola Zaïre (EBOV))], anticuerpo monoclonal quimérico; cadena pesada gamma1 (1-452) [Mus musculus VH (IGHV8-8*01(76.50%) -(IGHD) -IGHJ4*01) [ ] (1-122) -Homo sapiens IGHG1*01v, G1m17>G1m3, G1m1 (CH1 K120>R (219) ( ), bisagra ( ), CH2 ( ), CH3 D12 (361), L14 (363) ( ), CHS ( )) ( )], ( ')-disulfuro con la cadena ligera kappa (1'-213') [Mus musculus V-KAPPA (IGKV6-13*01 (94.70%) -IGKJ5*01) [6.3.9] (1'-106') -Homo sapiens IGKC*01, Km3 A45.1 (152), V101 (190) (107'-213')]; dímero ( '': '')-bisdisulfuro inmunomodulador, antiviral Heavy chain / Chaîne lourde /Cadena pesada DVKLLESGGG LVQPGGSLKL SCAASGFSLS TSGVGVGWFR QPSGKGLEWL 50 ALIWWDDDKY YNPSLKSQLS ISKDFSRNQV FLKISNVDIA DTATYYCARR 100 DPFGYDNAMG YWGQGTSVTV SSASTKGPSV FPLAPSSKST SGGTAALGCL 150 VKDYFPEPVT VSWNSGALTS GVHTFPAVLQ SSGLYSLSSV VTVPSSSLGT 200 QTYICNVNHK PSNTKVDKRV EPKSCDKTHT CPPCPAPELL GGPSVFLFPP 250 KPKDTLMISR TPEVTCVVVD VSHEDPEVKF NWYVDGVEVH NAKTKPREEQ 300 YNSTYRVVSV LTVLHQDWLN GKEYKCKVSN KALPAPIEKT ISKAKGQPRE 350 PQVYTLPPSR DELTKNQVSL TCLVKGFYPS DIAVEWESNG QPENNYKTTP 400 PVLDSDGSFF LYSKLTVDKS RWQQGNVFSC SVMHEALHNH YTQKSLSLSP 450 GK 452 Light chain / Chaîne légère / Cadena ligera DIVMTQSPLS LSTSVGDRVS LTCKASQNVG TAVAWYQQKP GQSPKLLIYS 50 ASNRYTGVPD RFTGSGSGTD FTLTISNMQS EDLADYFCQQ YSSYPLTFGA 100 GTKLELRTVA APSVFIFPPS DEQLKSGTAS VVCLLNNFYP REAKVQWKVD 150 NALQSGNSQE SVTEQDSKDS TYSLSSTLTL SKADYEKHKV YACEVTHQGL 200 SSPVTKSFNR GEC 213 Post-translational modifications Disulfide bridges location / Position des ponts disulfure / Posiciones de los puentes disulfuro Intra-H (C23-C104) ''-97'' 149''-205'' 266''-326'' 372''-430'' Intra-L (C23-C104) 23'-88' 133'-193' 23'''-88''' 133'''-193''' Inter-H-L (h 5-CL 126) ' 225''-213''' Inter-H-H (h 11, h 14) '' '' N-glycosylation sites / Sites de N-glycosylation / Posiciones de N-glicosilación H CH2 N84.4: 302, 302'' Complex bi-antennary (G0 > 75%) and high mannose (< 17%) Nicotiana benthamiana-type glycans / glycanes de type Nicotiana benthamiana bi-antennaires complexes (G0 > 75%) et riches en mannose (< 17%) / glicanos de tipo Nicotiana benthamiana biantenarios complejos (G0 > 75%) y alto contenido de manosa (< 17%). dasiglucagonum dasiglucagon mutated human glucagon analogue: [16-(2-methylalanine)(S>X),17-L-alanine(R>A),20-L-αglutamyl(Q>E),21-L-α-glutamyl(D>E),24-L-lysyl(Q>K),27-Lα-glutamyl(M>E),28-L-serine(N>S)]human glucagon glucagon analogue dasiglucagon analogue du glucagon humain muté : [16-(2-méthylalanine)(S>X),17-L-alanine(R>A),20-L-αglutamyl(Q>E),21-L-α-glutamyl(D>E),24-L-lysyl(Q>K),27-Lα-glutamyl(M>E),28-L-sérine(N>S)]glucagon humain analogue du glucagon 622
19 WHO Drug Information, Vol. 30, No. 4, 2016 Proposed INN: List 116 dasiglucagón análogo del glucagón humano mutado: [16-(2-metilalanina)(S>X),17-L-alanina(R>A),20-L-αglutamil(Q>E),21-L-α-glutamil(D>E),24-L-lisil(Q>K),27-L-αglutamil(M>E),28-L-serina(N>S)]glucagón humano análogo de glucagón C 153 H 225 N 43 O 49 S Sequence / Séquence / Secuencia HSQGTFTSDY SKYLDXARAE EFVKWLEST 29 Modified residue / Résidu modifié / Resto modificado X(16) 2-methylalanine (Aib) H 2 N CO 2 H (aminoisobutyric acid) H 3 C CH 3 delpazolidum delpazolid delpazolid delpazolid (5R)-3-[3-fluoro-4-(1-methyl-5,6-dihydro-1,2,4-triazin- 4(1H)-yl)phenyl]-5-(hydroxymethyl)-1,3-oxazolidin-2-one antibacterial (5R)-3-[3-fluoro-4-(1-méthyl-5,6-dihydro-1,2,4-triazin- 4(1H)-yl)phényl]-5-(hydroxyméthyl)-1,3-oxazolidin-2-one antibactérien (5R)-3-[3-fluoro-4-(1-metil-5,6-dihidro-1,2,4-triazin-4(1H)- il)fenil]-5-(hidroximetil)-1,3-oxazolidin-2-ona antibacteriano C 14 H 17 FN 4 O dematirsenum dematirsen all-p-ambo-2'-o-methyl-p-thioguanylyl-(3' 5')-2'-O- methyl-p-thiouridylyl-(3' 5')-2'-O-methyl-P-thiouridylyl- (3' 5')-2'-O-methyl-P-thioguanylyl-(3' 5')-2'-O,5-Cdimethyl-P-thiocytidylyl-(3' 5')-2'-O,5-C-dimethyl-Pthiocytidylyl-(3' 5')-2'-O-methyl-P-thiouridylyl-(3' 5')-2'- O,5-C-dimethyl-P-thiocytidylyl-(3' 5')-2'-O,5-C-dimethyl-P- thiocytidylyl-(3' 5')-2'-O-methyl-P-thioguanylyl-(3' 5')-2'- O-methyl-P-thioguanylyl-(3' 5')-2'-O-methyl-P-thiouridylyl- (3' 5')-2'-O-methyl-P-thiouridylyl-(3' 5')-2'-O,5-C- dimethyl-p-thiocytidylyl-(3' 5')-2'-O-methyl-P-thiouridylyl- (3' 5')-2'-O-methyl-P-thioguanylyl-(3' 5')-2'-O-methyl-P- thioadenylyl-(3' 5')-2'-O-methyl-P-thioadenylyl-(3' 5')-2'- O-methyl-P-thioguanylyl-(3' 5')-2'-O-methyl-P- thioguanylyl-(3' 5')-2'-O-methyl-P-thiouridylyl-(3' 5')-2'- O-methyl-P-thioguanylyl-(3' 5')-2'-O-methyl-P-thiouridylyl- (3' 5')-2'-O-methyl-P-thiouridylyl-(3' 5')-2'-O,5-Cdimethylcytidine promotion of functional dystrophin synthesis 623
20 Proposed INN: List 116 WHO Drug Information, Vol. 30, No. 4, 2016 dématirsen dematirsén tout-p-ambo-2'-o-méthyl-p-thioguanylyl-(3' 5')-2'-O- méthyl-p-thiouridylyl-(3' 5')-2'-O-méthyl-P-thiouridylyl- (3' 5')-2'-O-méthyl-P-thioguanylyl-(3' 5')-2'-O,5-Cdiméthyl-P-thiocytidylyl-(3' 5')-2'-O,5-C-diméthyl-Pthiocytidylyl-(3' 5')-2'-O-méthyl-P-thiouridylyl-(3' 5')-2'- O,5-C-diméthyl-P-thiocytidylyl-(3' 5')-2'-O,5-C-diméthyl-P- thiocytidylyl-(3' 5')-2'-O-méthyl-P-thioguanylyl-(3' 5')-2'- O-méthyl-P-thioguanylyl-(3' 5')-2'-O-méthyl-P-thiouridylyl- (3' 5')-2'-O-méthyl-P-thiouridylyl-(3' 5')-2'-O,5-C- diméthyl-p-thiocytidylyl-(3' 5')-2'-O-méthyl-P-thiouridylyl- (3' 5')-2'-O-méthyl-P-thioguanylyl-(3' 5')-2'-O-méthyl-P- thioadénylyl-(3' 5')-2'-O-méthyl-P-thioadénylyl-(3' 5')-2'- O-méthyl-P-thioguanylyl-(3' 5')-2'-O-méthyl-P- thioguanylyl-(3' 5')-2'-O-méthyl-P-thiouridylyl-(3' 5')-2'- O-méthyl-P-thioguanylyl-(3' 5')-2'-O-méthyl-P-thiouridylyl- (3' 5')-2'-O-méthyl-P-thiouridylyl-(3' 5')-2'-O,5-Cdiméthylcytidine stimulation de la synthèse de dystrophine fonctionnelle todo-p-ambo-2'-o-metil-p-tioguanilil-(3' 5')-2'-O-metil-Ptiouridilil-(3' 5')-2'-O-metil-P-tiouridilil-(3' 5')-2'-O-metil-Ptioguanilil-(3' 5')-2'-O,5-C-dimetil-P-tiocitidilil-(3' 5')-2'- O,5-C-dimetil-P-tiocitidilil-(3' 5')-2'-O-metil-P-tiouridilil- (3' 5')-2'-O,5-C-dimetil-P-tiocitidilil-(3' 5')-2'-O,5-C- dimetil-p-tiocitidilil-(3' 5')-2'-O-metil-P-tioguanilil-(3' 5')- 2'-O-metil-P-tioguanilil-(3' 5')-2'-O-metil-P-tiouridilil- (3' 5')-2'-O-metil-P-tiouridilil-(3' 5')-2'-O,5-C-dimetil-P- tiocitidilil-(3' 5')-2'-O-metil-P-tiouridilil-(3' 5')-2'-O-metil- P-tioguanilil-(3' 5')-2'-O-metil-P-tioadenilil-(3' 5')-2'-O- metil-p-tioadenilil-(3' 5')-2'-O-metil-P-tioguanilil-(3' 5')-2'- O-metil-P-tioguanilil-(3' 5')-2'-O-metil-P-tiouridilil-(3' 5')- 2'-O-metil-P-tioguanilil-(3' 5')-2'-O-metil-P-tiouridilil- (3' 5')-2'-O-metil-P-tiouridilil-(3' 5')-2'-O,5-Cdimetilcitidina estimulación de la síntesis de distrofina funcional C 266 H 354 N 86 O 156 P 24 S (3'-5')-(P-thio)[Gm-Um-Um-Gm-m5Cm-m5Cm-Um-m5Cm-m5Cm-Gm-Gm-Um-Um-m5Cm-Um- Gm-Am-Am-Gm-Gm-Um-Gm-Um-Um-m5Cm] Legend: m as suffix = 2'-O-methyl m5 as prefix = 5-C-methyl derazantinibum derazantinib dérazantinib derazantinib (6R)-6-(2-fluorophenyl)-N-(3-{2-[(2-methoxyethyl)amino] ethyl}phenyl)-5,6-dihydrobenzo[h]quinazolin-2-amine antineoplastic (6R)-6-(2-fluorophényl)-N-(3-{2-[(2-méthoxyéthyl)amino] éthyl}phényl)-5,6-dihydrobenzo[h]quinazolin-2-amine antinéoplasique (6R)-6-(2-fluorofenil)-N-(3-{2-[(2-metoxietil)amino]etil}fenil)- 5,6-dihidrobenzo[h]quinazolin-2-amina antineoplásico 624
21 WHO Drug Information, Vol. 30, No. 4, 2016 Proposed INN: List 116 C 29 H 29 FN 4 O dezapelisibum dezapelisib dézapélisib dezapelisib 6-(3-fluorophenyl)-3-methyl-7-[(1S)-1-(7H-purin- 6-ylamino)ethyl]-5H-[1,3]thiazolo[3,2-a]pyrimidin-5-one antineoplastic 6-(3-fluorophényl)-3-méthyl-7-[(1S)-1-(7H-purin- 6-ylamino)éthyl]-5H-[1,3]thiazolo[3,2-a]pyrimidin-5-one antinéoplasique 6-(3-fluorofenil)-3-metil-7-[(1S)-1-(7H-purin-6-ilamino)etil]- 5H-[1,3]tiazolo[3,2-a]pirimidin-5-ona antineoplásico C 20 H 16 FN 7 OS donaperminogenum seltoplasmidum # donaperminogene seltoplasmid donaperminogène seltoplasmide Plasmid DNA vector (pck) containing a genomic-cdna hybrid of the human hepatocyte growth factor (HGF) gene, HGF-X7, expressing two wild-type isoforms of HGF, HGF 723 and HGF 728, under the control of the promoter and enhancer of the immediate-early (IE) gene of the human cytomegalovirus (HCMV). gene therapy (angiogenesis stimulator) Vecteur constitué d'adn plasmidique (pck) contenant un hybride de l'adn génomique complémentaire (cdna) du gène du facteur de croissance des hépatocytes humain (HGF), HGF-7, qui exprime deux isoformes sauvages de HGF, HGF 723 et HGF 728, sous le contrôle du promoteur et activateur du gène immédiat-précoce du cytomégalovirus humain (CMV) thérapie génique (stimulateur de l'angiogénèse) 625
22 Proposed INN: List 116 WHO Drug Information, Vol. 30, No. 4, 2016 donapermingén seltoplásmido Vector de DNA plasmídico (pck) que contiene un híbrido de DNA genómico-dna complementario (cdna) del gen del factor de crecimiento de hepatocitos humano (HGF), HGF-X7, que expresa dos isoformas salvajes/silvestres de HGF, HGF 723 y HGF 728, bajo el control del promotor y el potenciador (enhancer) del gen inmediato-temprano (IE) del citomegalovirus humano terapia génica (estimulador de la angiogénesis) dorzagliatinum dorzagliatin dorzagliatine dorzagliatina (2S)-2-[4-(2-chlorophenoxy)-2-oxo-2,5-dihydro-1H-pyrrol- 1-yl]-N-{1-[(2R)-2,3-dihydroxypropyl]-1H-pyrazol-3-yl}- 4-methylpentanamide antidiabetic (2S)-2-[4-(2-chlorophénoxy)-2-oxo-2,5-dihydro-1H-pyrrol- 1-yl]-N-{1-[(2R)-2,3-dihydroxypropyl]-1H-pyrazol-3-yl}- 4-méthylpentanamide antidiabétique (2S)-2-[4-(2-clorofenoxi)-2-oxo-2,5-dihidro-1H-pirrol-1-il]- N-{1-[(2R)-2,3-dihidroxipropil]-1H-pirazol-3-il}- 4-metilpentanamida hipoglucemiante C 22 H 27 ClN 4 O dotinuradum dotinurad dotinurad (3,5-dichloro-4-hydroxyphenyl)(1,1-dioxo-1,2-dihydro- 3H-1λ 6-1,3-benzothiazol-3-yl)methanone urate transporter inhibitor (3,5-dichloro-4-hydroxyphényl)(1,1-dioxo-1,2-dihydro- 3H-1λ 6-1,3-benzothiazol-3-yl)méthanone inhibiteur du transporteur de l'urate 626
International Nonproprietary Names for Pharmaceutical Substances (INN)
 WH Drug Information, Vol. 30, No. 2, 2016 Proposed INN: List 115 International Nonproprietary Names for Pharmaceutical Substances (INN) Notice is hereby given that, in accordance with article 3 of the
WH Drug Information, Vol. 30, No. 2, 2016 Proposed INN: List 115 International Nonproprietary Names for Pharmaceutical Substances (INN) Notice is hereby given that, in accordance with article 3 of the
International Nonproprietary Names for Pharmaceutical Substances (INN)
 WHO Drug Information, Vol. 29, No. 4, 2015 Proposed INN: List 114 International Nonproprietary Names for Pharmaceutical Substances (INN) Notice is hereby given that, in accordance with article 3 of the
WHO Drug Information, Vol. 29, No. 4, 2015 Proposed INN: List 114 International Nonproprietary Names for Pharmaceutical Substances (INN) Notice is hereby given that, in accordance with article 3 of the
International Nonproprietary Names for Pharmaceutical Substances (INN)
 W Drug Information, Vol. 29, o. 2, 2015 Proposed I: List 113 International onproprietary ames for Pharmaceutical Substances (I) otice is hereby given that, in accordance with article 3 of the Procedure
W Drug Information, Vol. 29, o. 2, 2015 Proposed I: List 113 International onproprietary ames for Pharmaceutical Substances (I) otice is hereby given that, in accordance with article 3 of the Procedure
International Nonproprietary Names for Pharmaceutical Substances (INN)
 W Drug Information, Vol. 27, o. 2, 2013 Proposed I: List 109 International onproprietary ames for Pharmaceutical Substances (I) otice is hereby given that, in accordance with article 3 of the Procedure
W Drug Information, Vol. 27, o. 2, 2013 Proposed I: List 109 International onproprietary ames for Pharmaceutical Substances (I) otice is hereby given that, in accordance with article 3 of the Procedure
International Nonproprietary Names for Pharmaceutical Substances (INN)
 W Drug Information, Vol. 27, o. 4, 2013 Proposed I: List 110 International onproprietary ames for Pharmaceutical Substances (I) otice is hereby given that, in accordance with article 3 of the Procedure
W Drug Information, Vol. 27, o. 4, 2013 Proposed I: List 110 International onproprietary ames for Pharmaceutical Substances (I) otice is hereby given that, in accordance with article 3 of the Procedure
International Nonproprietary Names for Pharmaceutical Substances (INN)
 W Drug Information, Vol. 22, o. 2, 2008 Proposed I: List 99 International onproprietary ames for Pharmaceutical Substances (I) otice is hereby given that, in accordance with article 3 of the Procedure
W Drug Information, Vol. 22, o. 2, 2008 Proposed I: List 99 International onproprietary ames for Pharmaceutical Substances (I) otice is hereby given that, in accordance with article 3 of the Procedure
ENCL.: (1)* Prop. INN: List 111 published in WHO Drug Information, Vol. 28, No.2, 2014.
 0F P 20, AVEUE APPIA C-1211 GEEVA 27 SWITZERLAD TEL CETRAL +41 22 791 2111 FAX CETRAL +41 22 791 3111 WWW.W.IT Ref.: C.L.30.2014 The World ealth rganization presents its compliments and has the honour
0F P 20, AVEUE APPIA C-1211 GEEVA 27 SWITZERLAD TEL CETRAL +41 22 791 2111 FAX CETRAL +41 22 791 3111 WWW.W.IT Ref.: C.L.30.2014 The World ealth rganization presents its compliments and has the honour
International Nonproprietary Names for Pharmaceutical Substances (INN)
 W Drug Information, Vol. 24, o. 4, 2010 Proposed I: List 104 International onproprietary ames for Pharmaceutical Substances (I) otice is hereby given that, in accordance with article 3 of the Procedure
W Drug Information, Vol. 24, o. 4, 2010 Proposed I: List 104 International onproprietary ames for Pharmaceutical Substances (I) otice is hereby given that, in accordance with article 3 of the Procedure
International Nonproprietary Names for Pharmaceutical Substances (INN)
 W Drug Information, Vol. 28, o. 4, 2014 Proposed I: List 112 International onproprietary ames for Pharmaceutical Substances (I) otice is hereby given that, in accordance with article 3 of the Procedure
W Drug Information, Vol. 28, o. 4, 2014 Proposed I: List 112 International onproprietary ames for Pharmaceutical Substances (I) otice is hereby given that, in accordance with article 3 of the Procedure
International Nonproprietary Names for Pharmaceutical Substances (INN)
 W Drug Information Vol. 24, o. 2, 2010 Proposed I: List 103 International onproprietary ames for Pharmaceutical Substances (I) otice is hereby given that, in accordance with article 3 of the Procedure
W Drug Information Vol. 24, o. 2, 2010 Proposed I: List 103 International onproprietary ames for Pharmaceutical Substances (I) otice is hereby given that, in accordance with article 3 of the Procedure
International Nonproprietary Names for Pharmaceutical Substances (INN)
 W Drug Information, Vol. 25, o. 4, 2011 Proposed I: List 106 International onproprietary ames for Pharmaceutical Substances (I) otice is hereby given that, in accordance with article 3 of the Procedure
W Drug Information, Vol. 25, o. 4, 2011 Proposed I: List 106 International onproprietary ames for Pharmaceutical Substances (I) otice is hereby given that, in accordance with article 3 of the Procedure
International Nonproprietary Names for Pharmaceutical Substances (INN)
 W Drug Information, Vol. 23, o. 2, 2009 Proposed I: List 101 International onproprietary ames for Pharmaceutical Substances (I) otice is hereby given that, in accordance with article 3 of the Procedure
W Drug Information, Vol. 23, o. 2, 2009 Proposed I: List 101 International onproprietary ames for Pharmaceutical Substances (I) otice is hereby given that, in accordance with article 3 of the Procedure
MASTER. Zootecnia y Gestión Sostenible: Ganadería Ecológica e Integrada
 http://imgt.cines.fr/textes/imgtveterinary/ http://imgt.cines.fr/textes/imgteducation/ http://www.vetmed.wsu.edu/depts-moab/ ANTICUERPOS HUMANOS EN ANIMALES ANTICUERPOS HUMANOS EN ANIMALES Each of the
http://imgt.cines.fr/textes/imgtveterinary/ http://imgt.cines.fr/textes/imgteducation/ http://www.vetmed.wsu.edu/depts-moab/ ANTICUERPOS HUMANOS EN ANIMALES ANTICUERPOS HUMANOS EN ANIMALES Each of the
SISTEMA DE VINCULACION
 SISTEMA DE VINCULACION % REGULACION SANITARIA Y LA PROPIEDAD INDUSTRIAL Armando Valencia Hernández Coordinación de Patentes-Farmacéutica. Instituto Mexicano de la Propiedad Industrial armando.valencia@impi.gob.mx
SISTEMA DE VINCULACION % REGULACION SANITARIA Y LA PROPIEDAD INDUSTRIAL Armando Valencia Hernández Coordinación de Patentes-Farmacéutica. Instituto Mexicano de la Propiedad Industrial armando.valencia@impi.gob.mx
MEDIA : 72.14 PASS RATE: 60.6%
 12 Examen I 10 8 Frecuencia (%) 6 4 2 0 96 94 92 90 88 84 82 80 78 76 74 72 70 68 66 64 62 60 58 56 54 52 50 42 38 Puntuación (100) MEDIA : 72.14 PASS RATE: 60.6% 12 Examen I 10 Frecuencia (%) 8 6 4 ESTIMADO
12 Examen I 10 8 Frecuencia (%) 6 4 2 0 96 94 92 90 88 84 82 80 78 76 74 72 70 68 66 64 62 60 58 56 54 52 50 42 38 Puntuación (100) MEDIA : 72.14 PASS RATE: 60.6% 12 Examen I 10 Frecuencia (%) 8 6 4 ESTIMADO
International Nonproprietary Names for Pharmaceutical Substances (INN)
 W Drug Information, Vol. 23, o. 4, 2009 Proposed I: List 102 International onproprietary ames for Pharmaceutical Substances (I) otice is hereby given that, in accordance with article 3 of the Procedure
W Drug Information, Vol. 23, o. 4, 2009 Proposed I: List 102 International onproprietary ames for Pharmaceutical Substances (I) otice is hereby given that, in accordance with article 3 of the Procedure
CONSENT FOR HIV BLOOD TEST
 i have been informed that a sample of my blood will be obtained and tested to determine the presence of antibodies to human immunodeficiency Virus (hiv), the virus that causes Acquired immune Deficiency
i have been informed that a sample of my blood will be obtained and tested to determine the presence of antibodies to human immunodeficiency Virus (hiv), the virus that causes Acquired immune Deficiency
TECHNOLOGY ENHANCED LANGUAGE LEARNING MODULE Module on Las partes del cuerpo humano
 Student s name: TECHNOLOGY ENHANCED LANGUAGE LEARNING MODULE Module on Las partes del cuerpo humano INTERPRETIVE MODE: Preparation Phase: In this module, you will learn about Las partes del cuerpo humano.
Student s name: TECHNOLOGY ENHANCED LANGUAGE LEARNING MODULE Module on Las partes del cuerpo humano INTERPRETIVE MODE: Preparation Phase: In this module, you will learn about Las partes del cuerpo humano.
MASTER. Zootecnia y Gestión Sostenible: Ganadería Ecológica e Integrada. Inmunogenética.
 Dr. Diego Llanes Dra. Ángela Moreno Dr. José Pérez de la Lastra Inmunogenética. VARIABILIDAD DE LAS MOLÉCULAS RELACIONADAS CON EL SISTEMA INMUNE. PARTE DE LA GENÉTICA EN LA QUE SE USAN ANTICUERPOS PARA
Dr. Diego Llanes Dra. Ángela Moreno Dr. José Pérez de la Lastra Inmunogenética. VARIABILIDAD DE LAS MOLÉCULAS RELACIONADAS CON EL SISTEMA INMUNE. PARTE DE LA GENÉTICA EN LA QUE SE USAN ANTICUERPOS PARA
International Nonproprietary Names for Pharmaceutical Substances (INN)
 W Drug Information, Vol. 21, o. 2, 2007 Proposed I: List 97 International onproprietary ames for Pharmaceutical Substances (I) otice is hereby given that, in accordance with article 3 of the Procedure
W Drug Information, Vol. 21, o. 2, 2007 Proposed I: List 97 International onproprietary ames for Pharmaceutical Substances (I) otice is hereby given that, in accordance with article 3 of the Procedure
Blair Storage Bed / Lit avec Rangement / Cama con Almacenamiento - Queen, King
 English This page lists the contents included in the box. Please take time to identify the hardware as well as the individual components of the product. s you unpack and prepare for assembly, place the
English This page lists the contents included in the box. Please take time to identify the hardware as well as the individual components of the product. s you unpack and prepare for assembly, place the
Level 1 Spanish, 2013
 90911 909110 1SUPERVISOR S Level 1 Spanish, 2013 90911 Demonstrate understanding of a variety of Spanish texts on areas of most immediate relevance 9.30 am Tuesday 3 December 2013 Credits: Five Achievement
90911 909110 1SUPERVISOR S Level 1 Spanish, 2013 90911 Demonstrate understanding of a variety of Spanish texts on areas of most immediate relevance 9.30 am Tuesday 3 December 2013 Credits: Five Achievement
CERTIFICADO DE CUMPLIMIENTO DE NCF 1,2 / CERTIFICATE OF GMP COMPLIANCE OF A MANUFACTURER 1,2. Parte 1 / Part 1
 Certificado Nº / Certificate No: ES/072/15 CERTIFICADO DE CUMPLIMIENTO DE NCF 1,2 / CERTIFICATE OF GMP COMPLIANCE OF A MANUFACTURER 1,2 Parte 1 / Part 1 Emitido en virtud de una inspección según artículo
Certificado Nº / Certificate No: ES/072/15 CERTIFICADO DE CUMPLIMIENTO DE NCF 1,2 / CERTIFICATE OF GMP COMPLIANCE OF A MANUFACTURER 1,2 Parte 1 / Part 1 Emitido en virtud de una inspección según artículo
Catch and export quotas for specimens of Acipenseriformes species included in Appendix II for 2004
 Catch and export quotas for specimens of Acipenseriformes species included in Appendix II for 2004 de captura y exportación para especímenes de especies de Acipenseriformes incluidas en el Apéndice II
Catch and export quotas for specimens of Acipenseriformes species included in Appendix II for 2004 de captura y exportación para especímenes de especies de Acipenseriformes incluidas en el Apéndice II
MEMORANDUM DE ENTENDIMIENTO ENTRE EL GOBIERNO DE LA REPUBLICA ARGENTINA Y
 MEMORANDUM DE ENTENDIMIENTO ENTRE EL GOBIERNO DE LA REPUBLICA ARGENTINA Y EL GOBIERNO DE LA REPUBLICA DE COREA PARA EL ESTABLECIMIENTO DE UNA COMISION MIXTA El Gobierno de la República Argentina y el Gobierno
MEMORANDUM DE ENTENDIMIENTO ENTRE EL GOBIERNO DE LA REPUBLICA ARGENTINA Y EL GOBIERNO DE LA REPUBLICA DE COREA PARA EL ESTABLECIMIENTO DE UNA COMISION MIXTA El Gobierno de la República Argentina y el Gobierno
TD-500 MANUAL DE INSTRUCCIONES USER S MANUAL MANUEL D UTILISATION 7/ MI1516 -
 TD-500 MANUAL DE INSTRUCCIONES USER S MANUAL MANUEL D UTILISATION 7/2007-0 MI1516 - Combinador y Repartidor COFDM DVB-T TD-500 1. DESCRIPCIÓN FUNCIONAL El TD-500 es un combinador y repartidor DVB-T de
TD-500 MANUAL DE INSTRUCCIONES USER S MANUAL MANUEL D UTILISATION 7/2007-0 MI1516 - Combinador y Repartidor COFDM DVB-T TD-500 1. DESCRIPCIÓN FUNCIONAL El TD-500 es un combinador y repartidor DVB-T de
Health in Peru, 1991-2003. Prepared by Leigh Campoamor
 Prepared by Leigh Campoamor Princeton University Library Princeton, NJ 2003 Scope Note Contents: This collection contains pamphlets, articles, and other miscellaneous items addressing a range of health-related
Prepared by Leigh Campoamor Princeton University Library Princeton, NJ 2003 Scope Note Contents: This collection contains pamphlets, articles, and other miscellaneous items addressing a range of health-related
Janssen Prescription Assistance. www.janssenprescriptionassistance.com
 Janssen Prescription Assistance www.janssenprescriptionassistance.com Janssen Prescription Assistance What is Prescription Assistance? Prescription assistance programs provide financial help to people
Janssen Prescription Assistance www.janssenprescriptionassistance.com Janssen Prescription Assistance What is Prescription Assistance? Prescription assistance programs provide financial help to people
Estrategias de Inmunoterapia en Cáncer
 Estrategias de Inmunoterapia en Cáncer Carlos Parra-López. MD., PH.D Universidad Nacional de Colombia, Facultad de Medicina. Departamento de Microbiología Bogotá, Marzo 2014 El Sistema Inmune: Un sistema
Estrategias de Inmunoterapia en Cáncer Carlos Parra-López. MD., PH.D Universidad Nacional de Colombia, Facultad de Medicina. Departamento de Microbiología Bogotá, Marzo 2014 El Sistema Inmune: Un sistema
1) Through the left navigation on the A Sweet Surprise mini- site. Launch A Sweet Surprise Inicia Una dulce sorpresa 2016
 [[Version One (The user has not registered and is not logged in) Inicia Una dulce sorpresa 2016 To launch the Global Siddha Yoga Satsang for New Year s Day 2016, A Sweet Surprise, enter your username and
[[Version One (The user has not registered and is not logged in) Inicia Una dulce sorpresa 2016 To launch the Global Siddha Yoga Satsang for New Year s Day 2016, A Sweet Surprise, enter your username and
Verbs in the Present Tense (Los verbos en el tiempo presente) First conjugation: -ar
 Verbs in the Present Tense (Los verbos en el tiempo presente) First conjugation: -ar The fundamental parts of the Spanish verb The infinitive: The basic, unconjugated form, the one that corresponds to
Verbs in the Present Tense (Los verbos en el tiempo presente) First conjugation: -ar The fundamental parts of the Spanish verb The infinitive: The basic, unconjugated form, the one that corresponds to
Erradicación del VIH
 Erradicación del VIH Dr. Santiago Perez Patrigeon MD, PhD Departamento de Infectología Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán El paciente Berlin Timothy Roy Brown Hombre 40
Erradicación del VIH Dr. Santiago Perez Patrigeon MD, PhD Departamento de Infectología Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán El paciente Berlin Timothy Roy Brown Hombre 40
Beckett 5 Shelf Bookcase / Bibliothéque 5 Étagéres / 5 Estantería Librero
 English This page lists the contents included in the box. Please take time to identify the hardware as well as the individual components of the product. As you unpack and prepare for assembly, place the
English This page lists the contents included in the box. Please take time to identify the hardware as well as the individual components of the product. As you unpack and prepare for assembly, place the
REGISTRATION FOR ARNALDO CAPRAI GRUPPO TESSILE S.R.L. NEWSLETTER
 REGISTRATION FOR ARNALDO CAPRAI GRUPPO TESSILE S.R.L. NEWSLETTER Personal data processing notice This notice is given pursuant to article 13, legislative decree 196/2003 Personal Data Protection Code.
REGISTRATION FOR ARNALDO CAPRAI GRUPPO TESSILE S.R.L. NEWSLETTER Personal data processing notice This notice is given pursuant to article 13, legislative decree 196/2003 Personal Data Protection Code.
LAS VEGAS, NEVADA, USA JUNE 19-23, 2008 Tropicana Hotel and Casino
 Grand Prix - Sabre Feminin, Women s Sabre, Sable Femenino Grand Prix - Sabre Masculin, Men s Sabre, Sable Masculino Coupe Du Monde - Fleuret Feminin, Women s Foil, Florete Femenino LAS VEGAS, NEVADA, USA
Grand Prix - Sabre Feminin, Women s Sabre, Sable Femenino Grand Prix - Sabre Masculin, Men s Sabre, Sable Masculino Coupe Du Monde - Fleuret Feminin, Women s Foil, Florete Femenino LAS VEGAS, NEVADA, USA
HD Media Tower / Tour Hi-Fi / Medios de Torre
 English This page lists the contents included in the box. Please take time to identify the hardware as well as the individual components of the product. s you unpack and prepare for assembly, place the
English This page lists the contents included in the box. Please take time to identify the hardware as well as the individual components of the product. s you unpack and prepare for assembly, place the
Definiciones de Inmunología
 Definiciones de Inmunología Son sustancias extrañas a nuestro organismo que desencadenan la formación de Anticuerpos (Ac). La unión entre Ag y Ac es de naturaleza no covalente. Hay complementariedad entre
Definiciones de Inmunología Son sustancias extrañas a nuestro organismo que desencadenan la formación de Anticuerpos (Ac). La unión entre Ag y Ac es de naturaleza no covalente. Hay complementariedad entre
Cher client, Toute l équipe COMMENCAL vous remercie d avoir effectué votre commande.
 Cher client, Toute l équipe COMMENCAL vous remercie d avoir effectué votre commande. Vous avez fait le choix du paiement par virement bancaire. Afin de réaliser ce dernier, merci de bien vouloir respecter
Cher client, Toute l équipe COMMENCAL vous remercie d avoir effectué votre commande. Vous avez fait le choix du paiement par virement bancaire. Afin de réaliser ce dernier, merci de bien vouloir respecter
MANUAL EASYCHAIR. A) Ingresar su nombre de usuario y password, si ya tiene una cuenta registrada Ó
 MANUAL EASYCHAIR La URL para enviar su propuesta a la convocatoria es: https://easychair.org/conferences/?conf=genconciencia2015 Donde aparece la siguiente pantalla: Se encuentran dos opciones: A) Ingresar
MANUAL EASYCHAIR La URL para enviar su propuesta a la convocatoria es: https://easychair.org/conferences/?conf=genconciencia2015 Donde aparece la siguiente pantalla: Se encuentran dos opciones: A) Ingresar
HOMEWORK ASSIGNMENTS, TEST AND QUIZ DUE DATES: STUDY GUIDE and CLASS NOTES. NOV-04 TO NOV-22, 2016 SPANISH 2 PERIOD 7 S. DePastino
 HOMEWORK ASSIGNMENTS, TEST AND QUIZ DUE DATES: STUDY GUIDE and CLASS NOTES NOV-04 TO NOV-22, 2016 SPANISH 2 PERIOD 7 S. DePastino Unidad 2 Etapa 2 11-04 A TAREA: Estudiar el vocabulario libro p. 147 Iniciar
HOMEWORK ASSIGNMENTS, TEST AND QUIZ DUE DATES: STUDY GUIDE and CLASS NOTES NOV-04 TO NOV-22, 2016 SPANISH 2 PERIOD 7 S. DePastino Unidad 2 Etapa 2 11-04 A TAREA: Estudiar el vocabulario libro p. 147 Iniciar
Tema 5 Estructura cuaternaria de proteínas
 Tema 5 Estructura cuaternaria de proteínas Estructura y función de la Hemoglobina Unión de la Hemoglobina a ligandos Curva unión O 2 a la Hemoglobina Efectores alostéricos Efecto del ph sobre la saturación
Tema 5 Estructura cuaternaria de proteínas Estructura y función de la Hemoglobina Unión de la Hemoglobina a ligandos Curva unión O 2 a la Hemoglobina Efectores alostéricos Efecto del ph sobre la saturación
Análisis de la coestimulación vía CD28 en células linfoides de pacientes infectados. con el virus de la Hepatitis C RESUMEN
 Análisis de la coestimulación vía CD28 en células linfoides de pacientes infectados con el virus de la Hepatitis C RESUMEN La infección por el virus de la hepatitis C (VHC) afecta a más de 170 millones
Análisis de la coestimulación vía CD28 en células linfoides de pacientes infectados con el virus de la Hepatitis C RESUMEN La infección por el virus de la hepatitis C (VHC) afecta a más de 170 millones
Fun with infinitives
 Fun with infinitives Fun with Infinitives Infinitives in Spanish are unassigned actions that when translated into English always start with the word to. Spanish- CANTAR English- to sing Fun with Infinitives
Fun with infinitives Fun with Infinitives Infinitives in Spanish are unassigned actions that when translated into English always start with the word to. Spanish- CANTAR English- to sing Fun with Infinitives
OPERATING INSTRUCTIONS PIT JACK COD. 51105 COD. 51106 GUARANTEE... 7
 OPERATING INSTRUCTIONS PIT JACK COD. 51105 COD. 51106 GUARANTEE... 7 ENGLISH FORWARD MAIN SPECIFICATIONS MAIN CONSTRUCTION 2 PART LIST OF PIT JACK 3 4 PART LIST OF PIT JACK NOTES IMPORTANT! The maker will
OPERATING INSTRUCTIONS PIT JACK COD. 51105 COD. 51106 GUARANTEE... 7 ENGLISH FORWARD MAIN SPECIFICATIONS MAIN CONSTRUCTION 2 PART LIST OF PIT JACK 3 4 PART LIST OF PIT JACK NOTES IMPORTANT! The maker will
Mecanismos de evolución de los genomas. - Duplicación de dominios y alargamientos de genes. - Exon-shuffling - Genes quimera
 Mecanismos de evolución de los genomas - Duplicación de dominios y alargamientos de genes. - Exon-shuffling - Genes quimera Evolución del tamaño de los genes Contenido Introducción: mecanismos de amplificación
Mecanismos de evolución de los genomas - Duplicación de dominios y alargamientos de genes. - Exon-shuffling - Genes quimera Evolución del tamaño de los genes Contenido Introducción: mecanismos de amplificación
GENERALIDADES DE LA RESPUESTA INMUNE
 GENERALIDADES DE LA RESPUESTA INMUNE 1 BACTERIAS REPLICACION EXTRACELULAR REPLICACION INTRACELULAR Neumococo Mycobacterium tuberculosis VIRUS 2 Parásitos Helmintos Protozoos Taenia Tripanosoma Crusi Hongos
GENERALIDADES DE LA RESPUESTA INMUNE 1 BACTERIAS REPLICACION EXTRACELULAR REPLICACION INTRACELULAR Neumococo Mycobacterium tuberculosis VIRUS 2 Parásitos Helmintos Protozoos Taenia Tripanosoma Crusi Hongos
UNIT ONE: Vocabulary and grammar-verb To Be. UNIT TWO : grammar; simple present- present progressive
 CREATING YOUR BLOG ENGLISH A2 GENERAL OBJECTIVE: Define terminology and grammatical structure of the concepts of both classroom and basic English course A2 guidelines for the implementation of these in
CREATING YOUR BLOG ENGLISH A2 GENERAL OBJECTIVE: Define terminology and grammatical structure of the concepts of both classroom and basic English course A2 guidelines for the implementation of these in
Vivir con el mieloma: pronóstico y calidad de vida
 Vivir con el mieloma: pronóstico y calidad de vida Carlos Fernández de Larrea Unidad de Amiloidosis y Mieloma Servicio de Hematología Hospital Clínic / IDIBAPS Universitat de Barcelona Seminario para pacientes
Vivir con el mieloma: pronóstico y calidad de vida Carlos Fernández de Larrea Unidad de Amiloidosis y Mieloma Servicio de Hematología Hospital Clínic / IDIBAPS Universitat de Barcelona Seminario para pacientes
Training for women s baseball will be at the University of Toronto Scarborough, and will take place from July 15 to 19.
 BASEBALL Competition Information Baseball competition and training at the TORONTO 2015 Pan American Games will be held from July 6 to 26. The men s baseball competition and training, as well as the women
BASEBALL Competition Information Baseball competition and training at the TORONTO 2015 Pan American Games will be held from July 6 to 26. The men s baseball competition and training, as well as the women
AIBA Coaches Data Collection Form
 Please indicate the level of Coaches Course you are applying for: 1Star Course DIGITAL PHOTO PHOTO DIGITALE FOTOGRAFÍA DIGITAL First Name / Prénom / Nombre Surname / Nom de Famille / Apellido(s) Nationality
Please indicate the level of Coaches Course you are applying for: 1Star Course DIGITAL PHOTO PHOTO DIGITALE FOTOGRAFÍA DIGITAL First Name / Prénom / Nombre Surname / Nom de Famille / Apellido(s) Nationality
FAMILY INDEPENDENCE ADMINISTRATION Seth W. Diamond, Executive Deputy Commissioner
 FAMILY INDEPENDENCE ADMINISTRATION Seth W. Diamond, Executive Deputy Commissioner James K. Whelan, Deputy Commissioner Policy, Procedures, and Training Lisa C. Fitzpatrick, Assistant Deputy Commissioner
FAMILY INDEPENDENCE ADMINISTRATION Seth W. Diamond, Executive Deputy Commissioner James K. Whelan, Deputy Commissioner Policy, Procedures, and Training Lisa C. Fitzpatrick, Assistant Deputy Commissioner
Moléculas Orgánicas. Capítulo 4: Bases Químicas de la Vida Compuestos Orgánicos. Cadenas. Biol 3051 Capítulo 4 Grupos Funcionales 07/09/2016
 Capítulo 4: Bases Químicas de la Vida Compuestos Orgánicos Figure 4.5a Cadenas (a) Varían en largo Dr. Fernando J. Bird-Picó Departamento de Biología Recinto Universitario de Mayagüez Etano Propano Figure
Capítulo 4: Bases Químicas de la Vida Compuestos Orgánicos Figure 4.5a Cadenas (a) Varían en largo Dr. Fernando J. Bird-Picó Departamento de Biología Recinto Universitario de Mayagüez Etano Propano Figure
CONTROLADORA PARA PIXELS CONPIX
 The LedEdit Software Instructions 1, Install the software to PC and open English version: When we installed The LedEdit Software, on the desktop we can see following icon: Please Double-click it, then
The LedEdit Software Instructions 1, Install the software to PC and open English version: When we installed The LedEdit Software, on the desktop we can see following icon: Please Double-click it, then
Ministerio de Salud Secretaría de Políticas, Regulación e Institutos A.N.M.A.T. Instituto Nacional de Medicamentos
 Certificado de Liberación de Lote de Producto Farmacéutico Fabricado para Exportación Batch Liberation Certificate of Medicaments Manufactured for Export El INSTITUTO NACIONAL DE MEDICAMENTOS, organismo
Certificado de Liberación de Lote de Producto Farmacéutico Fabricado para Exportación Batch Liberation Certificate of Medicaments Manufactured for Export El INSTITUTO NACIONAL DE MEDICAMENTOS, organismo
V.- V.-El El manejo de de las las Interrupciones
 Las Las V.- V.-El El manejo de de las las Conceptos Conceptos BásicosB Básicos Modos Modos de de Manejo Manejo Ejemplos Ejemplos de de aplicación aplicación Las Las El manejo de las en el 8051 Las interrupciones
Las Las V.- V.-El El manejo de de las las Conceptos Conceptos BásicosB Básicos Modos Modos de de Manejo Manejo Ejemplos Ejemplos de de aplicación aplicación Las Las El manejo de las en el 8051 Las interrupciones
Brain Tumors. Glioma Tumors
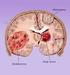 Brain Tumors A tumor that starts and grows in the brain is called a primary brain tumor. Primary brain tumors get their name from the type of cell or the part of the brain where they start growing. A secondary
Brain Tumors A tumor that starts and grows in the brain is called a primary brain tumor. Primary brain tumors get their name from the type of cell or the part of the brain where they start growing. A secondary
Sheridan Kitchen Cart / Cuisine Panier / Cocina Cesta
 English This page lists the contents included in the box. Please take time to identify the hardware as well as the individual components of the product. s you unpack and prepare for assembly, place the
English This page lists the contents included in the box. Please take time to identify the hardware as well as the individual components of the product. s you unpack and prepare for assembly, place the
ALBAYT BEACH - GOLF PACKAGES 2015 COSTA DEL SOL, front line beach holiday apartments
 ALBAYT BEACH - GOLF PACKAGES 2015 COSTA DEL SOL, front line beach holiday apartments ALBAYT PLATINUM GOLF PACKAGE: GF to choose from: San Roque New, Alcaidesa Golf, El Chaparral, El Paraíso Golf, FINCA
ALBAYT BEACH - GOLF PACKAGES 2015 COSTA DEL SOL, front line beach holiday apartments ALBAYT PLATINUM GOLF PACKAGE: GF to choose from: San Roque New, Alcaidesa Golf, El Chaparral, El Paraíso Golf, FINCA
Agentes Antivirales Los virus
 Los virus Los virus son elementos genéticos conteniendo AD o AR que pueden encontrarse alternadamente en dos estados: extracelular y intracelular. Los virus se apropian y reacondicionan la maquinaria biosintética
Los virus Los virus son elementos genéticos conteniendo AD o AR que pueden encontrarse alternadamente en dos estados: extracelular y intracelular. Los virus se apropian y reacondicionan la maquinaria biosintética
Texas Minimum State Vaccine Requirements for Child-Care Facilities
 2016-2017 Texas Minimum State Vaccine Requirements for Child-Care Facilities This chart summarizes the vaccine requirements incorporated in the Texas Administrative Code (TAC), Title 25 Health Services,
2016-2017 Texas Minimum State Vaccine Requirements for Child-Care Facilities This chart summarizes the vaccine requirements incorporated in the Texas Administrative Code (TAC), Title 25 Health Services,
SITUACIÓN ACTUAL DE LAS ENFERMEDADES MINORITARIAS: Qué hemos conseguido hasta ahora?
 SITUACIÓN ACTUAL DE LAS ENFERMEDADES MINORITARIAS: Qué hemos conseguido hasta ahora? Josep Torrent-Farnell Fundació Doctor Robert UAB Comité Medicamentos Huérfanos (COMP) Agencia Europea del Medicamento
SITUACIÓN ACTUAL DE LAS ENFERMEDADES MINORITARIAS: Qué hemos conseguido hasta ahora? Josep Torrent-Farnell Fundació Doctor Robert UAB Comité Medicamentos Huérfanos (COMP) Agencia Europea del Medicamento
Biosíntesis de Ácidos nucleicos (ARN) y Proteínas
 Biosíntesis de Ácidos nucleicos (ARN) y Proteínas Núcleo celular Dogma central de la Biología Molecular Repasando los Ácidos nucleicos. Empaquetamiento de ADN 46 cromosomas en las células somáticas del
Biosíntesis de Ácidos nucleicos (ARN) y Proteínas Núcleo celular Dogma central de la Biología Molecular Repasando los Ácidos nucleicos. Empaquetamiento de ADN 46 cromosomas en las células somáticas del
Guapo Using Ser and Tener to Describe People
 Guapo Using Ser and Tener to Describe People This document teaches the central piece of grammar in Guapo how to describe people using the verbs ser and tener as seen in these lyrics: Soy guapo. Tiene ojos
Guapo Using Ser and Tener to Describe People This document teaches the central piece of grammar in Guapo how to describe people using the verbs ser and tener as seen in these lyrics: Soy guapo. Tiene ojos
Ingeniería Genética en Eucariontes
 Universidad de Chile Facultad de Ciencias Químicas y Farmacéuticas Departamento de Bioquímica y Biología Molecular l Ingeniería Genética en Eucariontes Bibliografía : Recombinant DNA, Third Edition: Genes
Universidad de Chile Facultad de Ciencias Químicas y Farmacéuticas Departamento de Bioquímica y Biología Molecular l Ingeniería Genética en Eucariontes Bibliografía : Recombinant DNA, Third Edition: Genes
ELEVADOR HIDRÁULICO TROLLEY JACK
 MANUAL DE INSTRUCCIONES OPERATING INSTRUCTIONS ELEVADOR HIDRÁULICO TROLLEY JACK COD. 51101 ESPAÑOL... 2 ENGLISH... 6 GARANTIA / GUARANTEE... 9 ESPAÑOL INSTRUCCIONES DE USO IMPORTANTE: Ocasionalmente, durante
MANUAL DE INSTRUCCIONES OPERATING INSTRUCTIONS ELEVADOR HIDRÁULICO TROLLEY JACK COD. 51101 ESPAÑOL... 2 ENGLISH... 6 GARANTIA / GUARANTEE... 9 ESPAÑOL INSTRUCCIONES DE USO IMPORTANTE: Ocasionalmente, durante
 Instalación rápida Antes de proceder con la instalación, es importante que sepa: Una instalación completa incluye "Drivers" y "Programa", ambos elementos se pueden instalar fácilmente desde el CD del software.
Instalación rápida Antes de proceder con la instalación, es importante que sepa: Una instalación completa incluye "Drivers" y "Programa", ambos elementos se pueden instalar fácilmente desde el CD del software.
Inmunidad Adaptativa (adquirida ó específica)
 Inmunidad Adaptativa (adquirida ó específica) Procesamiento y presentación del Antígeno Receptores de Antígeno (BCR y TCR) Moléculas del Complejo Mayor de Histocompatibilidad Dra Silvina E. Gutiérrez Departamento
Inmunidad Adaptativa (adquirida ó específica) Procesamiento y presentación del Antígeno Receptores de Antígeno (BCR y TCR) Moléculas del Complejo Mayor de Histocompatibilidad Dra Silvina E. Gutiérrez Departamento
Adquisición Chubb Security Services
 Adquisición Chubb Security Services 16 Diciembre, 2013 1 Perfil de la Compañía Descripción de la compañía Presencia geográfica Constituida en 1972 Chubb Security Services Pty Limited: #2 en el mercado
Adquisición Chubb Security Services 16 Diciembre, 2013 1 Perfil de la Compañía Descripción de la compañía Presencia geográfica Constituida en 1972 Chubb Security Services Pty Limited: #2 en el mercado
Steps to Understand Your Child s Behavior. Customizing the Flyer
 Steps to Understand Your Child s Behavior Customizing the Flyer Hello! Here is the PDF Form Template for use in advertising Steps to Understanding Your Child s Behavior (HDS Behavior Level 1B). Because
Steps to Understand Your Child s Behavior Customizing the Flyer Hello! Here is the PDF Form Template for use in advertising Steps to Understanding Your Child s Behavior (HDS Behavior Level 1B). Because
74 Prime Time. conjetura Suposición acerca de un patrón o relación, basada en observaciones.
 A abundant number A number for which the sum of all its proper factors is greater than the number itself. For example, 24 is an abundant number because its proper factors, 1, 2, 3, 4, 6, 8, and 12, add
A abundant number A number for which the sum of all its proper factors is greater than the number itself. For example, 24 is an abundant number because its proper factors, 1, 2, 3, 4, 6, 8, and 12, add
Rhode Island Department of Health Three Capitol Hill Providence, RI 02908-5094
 Rhode Island Department of Health Three Capitol Hill Providence, RI 02908-5094 www.health.ri.gov Date: December 30, 2009 To: Parents and guardians of school-aged children in Rhode Island From: Director
Rhode Island Department of Health Three Capitol Hill Providence, RI 02908-5094 www.health.ri.gov Date: December 30, 2009 To: Parents and guardians of school-aged children in Rhode Island From: Director
IMMIGRATION Canada. Temporary Resident Visa. Mexico City Visa Office Instructions. Table of Contents IMM 5878 E (10-2015)
 IMMIGRATION Canada Table of Contents Document Checklist Temporary resident visa (available in Spanish) Emergency Processing Request Form Temporary Resident Visa Mexico City Visa Office Instructions This
IMMIGRATION Canada Table of Contents Document Checklist Temporary resident visa (available in Spanish) Emergency Processing Request Form Temporary Resident Visa Mexico City Visa Office Instructions This
PELICULAS CLAVES DEL CINE DE CIENCIA FICCION LOS DIRECTORES LOS ACTORES LOS ARGUMENTOS Y LAS ANECD
 PELICULAS CLAVES DEL CINE DE CIENCIA FICCION LOS DIRECTORES LOS ACTORES LOS ARGUMENTOS Y LAS ANECD 8 Feb, 2016 PCDCDCFLDLALAYLAHARG-PDF33-0 File 4,455 KB 96 Page If you want to possess a one-stop search
PELICULAS CLAVES DEL CINE DE CIENCIA FICCION LOS DIRECTORES LOS ACTORES LOS ARGUMENTOS Y LAS ANECD 8 Feb, 2016 PCDCDCFLDLALAYLAHARG-PDF33-0 File 4,455 KB 96 Page If you want to possess a one-stop search
Los monómeros de las proteínas
 Las proteínas Los monómeros de las proteínas El carbono α de los aminoácidos es asimétrico y por eso pueden darse dos formas isoméricas que se distinguen como D y L. Los seres vivos sólo construyen a.a.
Las proteínas Los monómeros de las proteínas El carbono α de los aminoácidos es asimétrico y por eso pueden darse dos formas isoméricas que se distinguen como D y L. Los seres vivos sólo construyen a.a.
VECTORES. MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 VECTORES 1) If A + B = C and their magnitudes are given by A + B = C, then the vectors A and B are oriented 1) A) parallel to each other (in the same direction). B) perpendicular relative to one other.
VECTORES 1) If A + B = C and their magnitudes are given by A + B = C, then the vectors A and B are oriented 1) A) parallel to each other (in the same direction). B) perpendicular relative to one other.
BEGINNING BAND PRACTICE JOURNAL #3 Also available online
 BEGINNING BAND PRACTICE JOURNAL #3 Also available online Name Date: the week of November 7th, 2016. Please record your practice time every day and turn in the journal, signed by a parent, Tuesday, November
BEGINNING BAND PRACTICE JOURNAL #3 Also available online Name Date: the week of November 7th, 2016. Please record your practice time every day and turn in the journal, signed by a parent, Tuesday, November
TELEVISOR A COLORES MANUAL DE SERVICIO MODELO : CP-29C40P. ATENCIÓN Antes de dar servicio al chasis, lea las PRECAUCIONES DE SEGURIDAD en este manual.
 LG TELEVISOR A COLORES MANUAL DE SERVICIO CHASIS : MC-53A MODELO : CP-29C40P ATENCIÓN Antes de dar servicio al chasis, lea las PRECAUCIONES DE SEGURIDAD en este manual. - 1 - - 2 - - 3 - - 4 - - 1 - -
LG TELEVISOR A COLORES MANUAL DE SERVICIO CHASIS : MC-53A MODELO : CP-29C40P ATENCIÓN Antes de dar servicio al chasis, lea las PRECAUCIONES DE SEGURIDAD en este manual. - 1 - - 2 - - 3 - - 4 - - 1 - -
Itziar Astigarraga/Mª Dolores Boyano Servicio de Pediatría. HU Cruces. UPV/EHU Departamento Biología Celular. UPV/EHU
 Estudio de las alteraciones inmunes en las histiocitosis y su aplicación en la búsqueda de biomarcadores para sepsis graves y nuevas terapias frente a leucemias Itziar Astigarraga/Mª Dolores Boyano Servicio
Estudio de las alteraciones inmunes en las histiocitosis y su aplicación en la búsqueda de biomarcadores para sepsis graves y nuevas terapias frente a leucemias Itziar Astigarraga/Mª Dolores Boyano Servicio
Screener for Peer Supporters
 Screener for Peer Supporters Primary Recruiter: Secondary Recruiter: Potential Peer Supporter Name: Phone #1: Home/Cell Phone #2: Home/Cell Address: City: Zip: Contact 1: Date: / / Contact 2: Date: / /
Screener for Peer Supporters Primary Recruiter: Secondary Recruiter: Potential Peer Supporter Name: Phone #1: Home/Cell Phone #2: Home/Cell Address: City: Zip: Contact 1: Date: / / Contact 2: Date: / /
Nomad. DESIGN BY I+D+i FORMA 5
 Nomad DESIGN BY I+D+i FORMA 5 Familia Nomad Nomad es la apuesta de Forma 5 para colectividades, especialmente ideado para dar soluciones funcionales a aulas de formación, salas de visionado, zonas de
Nomad DESIGN BY I+D+i FORMA 5 Familia Nomad Nomad es la apuesta de Forma 5 para colectividades, especialmente ideado para dar soluciones funcionales a aulas de formación, salas de visionado, zonas de
Radiación Externa. External Radiation. Radiación Interna. Internal Radiation
 Terapia de Radiación Radiation Therapy Spanish Translation Radiation therapy is sometimes called radiotherapy, x-ray therapy, or irradiation. It treats cancer by using beams of high- energy waves or streams
Terapia de Radiación Radiation Therapy Spanish Translation Radiation therapy is sometimes called radiotherapy, x-ray therapy, or irradiation. It treats cancer by using beams of high- energy waves or streams
International Centre for Settlement of Investment Disputes Washington, D.C. In the proceedings between
 International Centre for Settlement of Investment Disputes Washington, D.C. In the proceedings between Aguas Provinciales de Santa Fe S.A, Suez, Sociedad General de Aguas de Barcelona, S.A., and InterAguas
International Centre for Settlement of Investment Disputes Washington, D.C. In the proceedings between Aguas Provinciales de Santa Fe S.A, Suez, Sociedad General de Aguas de Barcelona, S.A., and InterAguas
TEMA 19: TOLERANCIA INMUNOLÓGICA. Concepto y desarrollo histórico. Tolerancia central y periférica. Tolerancia materno-fetal.
 TEMA 19: TOLERANCIA INMUNOLÓGICA. Concepto y desarrollo histórico. Tolerancia central y periférica. Tolerancia materno-fetal. OBJETIVOS - Conocer y distinguir los conceptos de tolerancia e ignorancia inmunológicas.
TEMA 19: TOLERANCIA INMUNOLÓGICA. Concepto y desarrollo histórico. Tolerancia central y periférica. Tolerancia materno-fetal. OBJETIVOS - Conocer y distinguir los conceptos de tolerancia e ignorancia inmunológicas.
Controles Habrá controles sobre los episodios de La comunidad y sobre las lecturas.
 ESPAÑOL 5M (5 UNITS) T/TH 1:00 - PM UCSC SUMMER 2015 INSTRUCTOR: Álvaro Romero EMAIL: romero@ucsc.edu OFFICE LOCATION: Hum 1, 134 ATENCIÓN AL ESTUDIANTE: Con cita previa Descripción del curso Este curso
ESPAÑOL 5M (5 UNITS) T/TH 1:00 - PM UCSC SUMMER 2015 INSTRUCTOR: Álvaro Romero EMAIL: romero@ucsc.edu OFFICE LOCATION: Hum 1, 134 ATENCIÓN AL ESTUDIANTE: Con cita previa Descripción del curso Este curso
TU EMBARAZO Y EL NACIMIENTO DEL BEBE GUIA PARA ADOLESCENTES EMBARAZADAS TEEN PREGNANCY AND PARENTI
 TU EMBARAZO Y EL NACIMIENTO DEL BEBE GUIA PARA ADOLESCENTES EMBARAZADAS TEEN PREGNANCY AND PARENTI 8 Feb, 2016 TEYENDBGPAETPAPWWET-PDF33-0 File 4,455 KB 96 Page If you want to possess a one-stop search
TU EMBARAZO Y EL NACIMIENTO DEL BEBE GUIA PARA ADOLESCENTES EMBARAZADAS TEEN PREGNANCY AND PARENTI 8 Feb, 2016 TEYENDBGPAETPAPWWET-PDF33-0 File 4,455 KB 96 Page If you want to possess a one-stop search
For Repair Parts, call
 Dayton Operating Instructions, Performance Specifications and Parts Manuals Models: NXZA thru NXZ9A; NYC0A thru NYC9A NYA0A thru NYA9A For Repair Parts, call -00--00 hours a day days a year Please provide
Dayton Operating Instructions, Performance Specifications and Parts Manuals Models: NXZA thru NXZ9A; NYC0A thru NYC9A NYA0A thru NYA9A For Repair Parts, call -00--00 hours a day days a year Please provide
United States Spain Treaties in Force
 Alien Amateur Radio Operators Agreement effected by exchange of notes Signed at Madrid December 11 and 20, 1979; Entered into force December 20, 1979. TIAS 9721 STATUS: Agreement effected by exchange of
Alien Amateur Radio Operators Agreement effected by exchange of notes Signed at Madrid December 11 and 20, 1979; Entered into force December 20, 1979. TIAS 9721 STATUS: Agreement effected by exchange of
Get an early start. Read this first. Use these Back-to-School flyers to reach parents early in the school year.
 Get an early start. Read this first. Use these Back-to-School flyers to reach parents early in the school year. Choose your favorite style, complete the form, then make enough copies to distribute them
Get an early start. Read this first. Use these Back-to-School flyers to reach parents early in the school year. Choose your favorite style, complete the form, then make enough copies to distribute them
REGULACIÓN GENÉTICA EN CÉLULAS EUCARIOTAS
 REGULACIÓN GENÉTICA EN CÉLULAS EUCARIOTAS INFORMACIÓN GENÉTICA GENOMA TRANSCRIPTOMA PROTEOMA CONTROL DE LA EXPRESIÓN GENÉTICA En un organismo multicelular existen muchos tipos celulares (diferenciación)
REGULACIÓN GENÉTICA EN CÉLULAS EUCARIOTAS INFORMACIÓN GENÉTICA GENOMA TRANSCRIPTOMA PROTEOMA CONTROL DE LA EXPRESIÓN GENÉTICA En un organismo multicelular existen muchos tipos celulares (diferenciación)
Estación Uno: Date, Weather, and How you feel
 Estación Uno: Date, Weather, and How you feel Learning Target: We are learning to write the date, talk about the weather outside, and state how we feel.. Criteria for Success: I can write the date in Spanish.
Estación Uno: Date, Weather, and How you feel Learning Target: We are learning to write the date, talk about the weather outside, and state how we feel.. Criteria for Success: I can write the date in Spanish.
Level 2 Spanish, 2010
 9 0 4 2 6 L P 2 Level 2 Spanish, 2010 90426 Listen to and understand spoken language in Spanish in less familiar contexts Credits: Six 2.00 pm Friday 26 November 2010 LISTENING PASSAGE BOOKLET This booklet
9 0 4 2 6 L P 2 Level 2 Spanish, 2010 90426 Listen to and understand spoken language in Spanish in less familiar contexts Credits: Six 2.00 pm Friday 26 November 2010 LISTENING PASSAGE BOOKLET This booklet
How to play CONTINUO For individual play and basic rules
 How to play CONTINUO For individual play and basic rules E1 Shuffle and place pack face down. Then simply take one card at a time from the top of the pack and place it face up on a flat surface touching
How to play CONTINUO For individual play and basic rules E1 Shuffle and place pack face down. Then simply take one card at a time from the top of the pack and place it face up on a flat surface touching
General Certificate of Education Advanced Subsidiary Examination June 2014
 General Certificate of Education Advanced Subsidiary Examination June 2014 Spanish Unit 2 Speaking Test Candidate s Material To be conducted by the teacher examiner between 7 March and 15 May 2014 (SPA2T)
General Certificate of Education Advanced Subsidiary Examination June 2014 Spanish Unit 2 Speaking Test Candidate s Material To be conducted by the teacher examiner between 7 March and 15 May 2014 (SPA2T)
Following are the form numbers of successful candidates:- (Syed Abrar Ali) REGISTRAR. Page 1 of 8
 0201 CE 0207 CE 0208 EE 0210 CS 0211 BI 0215 EL 0217 CE 0219 BE 0220 BE 0222 EE 0223 SE 0224 EE 0226 CS 0228 CS 0235 CS 0237 EE 0238 SE 0239 CI 0240 EL 0243 CI 0244 SE 0245 CE 0246 CS 0252 CI 0253 EE 0254
0201 CE 0207 CE 0208 EE 0210 CS 0211 BI 0215 EL 0217 CE 0219 BE 0220 BE 0222 EE 0223 SE 0224 EE 0226 CS 0228 CS 0235 CS 0237 EE 0238 SE 0239 CI 0240 EL 0243 CI 0244 SE 0245 CE 0246 CS 0252 CI 0253 EE 0254
III CONGRESO NACIONAL DE ATENCIÓN FARMACÉUTICA
 Adherencia a la terapia antirretroviral en pacientes infectados con el virus de la inmunodeficiencia humana que asisten a una clínica universitaria de enfermedades infecciosas en Venezuela. III CONGRESO
Adherencia a la terapia antirretroviral en pacientes infectados con el virus de la inmunodeficiencia humana que asisten a una clínica universitaria de enfermedades infecciosas en Venezuela. III CONGRESO
Paper Reference. Paper Reference(s) 6811/01 Edexcel GCE Spanish Advanced Subsidiary/Advanced Unit 1 Listening and Writing
 Centre No. Candidate No. Paper Reference 6 8 1 1 0 1 Paper Reference(s) 6811/01 Edexcel GCE Spanish Advanced Subsidiary/Advanced Unit 1 Listening and Writing Tuesday 19 May 2009 Morning Time: 1 hour Materials
Centre No. Candidate No. Paper Reference 6 8 1 1 0 1 Paper Reference(s) 6811/01 Edexcel GCE Spanish Advanced Subsidiary/Advanced Unit 1 Listening and Writing Tuesday 19 May 2009 Morning Time: 1 hour Materials
Características Técnicas Technical specifications
 serie concept prcl Concept 30 x 60 cm 12 x 23.6 60 x 60 cm 23.6 x 23.6 45 x 90 cm 17.7 x 35.4 Características Técnicas Technical specifications Gres porcelánico esmaltado Porcelain tiles Grupo Group: Bla
serie concept prcl Concept 30 x 60 cm 12 x 23.6 60 x 60 cm 23.6 x 23.6 45 x 90 cm 17.7 x 35.4 Características Técnicas Technical specifications Gres porcelánico esmaltado Porcelain tiles Grupo Group: Bla
Following are the form numbers of successful candidates:- (Dr. Mumtaz-Ul-Imam) REGISTRAR. Page 1 of 7
 0103 TE 0104 CE 0107 CE 0108 EE 0109 BE 0110 BE 0111 EL 0112 EE 0114 CS 0115 CS 0116 TE 0117 BE 0118 BE 0120 TE 0121 EE 0128 CS 0129 CE 0130 SE 0133 TE 0134 SE 0136 BE 0140 EE 0141 EE 0143 SE 0144 SE 0145
0103 TE 0104 CE 0107 CE 0108 EE 0109 BE 0110 BE 0111 EL 0112 EE 0114 CS 0115 CS 0116 TE 0117 BE 0118 BE 0120 TE 0121 EE 0128 CS 0129 CE 0130 SE 0133 TE 0134 SE 0136 BE 0140 EE 0141 EE 0143 SE 0144 SE 0145
Citotoxicidad mediada por células. Débora E. Aldana Salguero MD, PhD
 Citotoxicidad mediada por células Débora E. Aldana Salguero MD, PhD Las reacciones citotóxicas son sistemas efectores que se dirigen contra todas las células nucleadas. La respuesta generada por estas
Citotoxicidad mediada por células Débora E. Aldana Salguero MD, PhD Las reacciones citotóxicas son sistemas efectores que se dirigen contra todas las células nucleadas. La respuesta generada por estas
TABLA 1- Desplazamientos químicos (δ) de protones de metilos, metilenos y metinos de alcanos monosustituidos con sustituyentes x en posición α
 TABLA 1- Desplazamientos químicos (δ) de protones de metilos, metilenos y metinos de alcanos monosustituidos con sustituyentes x en posición α alógeno N S --N X 3 X 2 X - 3,- 2-0,86 0,91 1,25 1,33 1,50
TABLA 1- Desplazamientos químicos (δ) de protones de metilos, metilenos y metinos de alcanos monosustituidos con sustituyentes x en posición α alógeno N S --N X 3 X 2 X - 3,- 2-0,86 0,91 1,25 1,33 1,50
